Megabat: Difference between revisions
→Predators and parasites: | Add: year, pages, issue, volume, journal, title, pmc, pmid, author pars. 1-5. Formatted dashes. | You can use this tool yourself. Report bugs here. |
|||
| Line 230: | Line 230: | ||
The [[saltwater crocodile]], a native species, is a known predator of megabats, based on analysis of crocodile stomach contents in northern Australia.<ref>{{cite journal|doi=10.1371/journal.pone.0197159|pmid=29874276|pmc=5991389|title=Estuarine crocodiles in a tropical coastal floodplain obtain nutrition from terrestrial prey|journal=PLOS ONE|volume=13|issue=6|pages=e0197159|year=2018|last1=Adame|first1=Maria Fernanda|last2=Jardine|first2=Timothy D.|last3=Fry|first3=Brian|last4=Valdez|first4=Dominic|last5=Lindner|first5=Garry|last6=Nadji|first6=Jonathan|last7=Bunn|first7=Stuart E.|bibcode=2018PLoSO..1397159A}}</ref> |
The [[saltwater crocodile]], a native species, is a known predator of megabats, based on analysis of crocodile stomach contents in northern Australia.<ref>{{cite journal|doi=10.1371/journal.pone.0197159|pmid=29874276|pmc=5991389|title=Estuarine crocodiles in a tropical coastal floodplain obtain nutrition from terrestrial prey|journal=PLOS ONE|volume=13|issue=6|pages=e0197159|year=2018|last1=Adame|first1=Maria Fernanda|last2=Jardine|first2=Timothy D.|last3=Fry|first3=Brian|last4=Valdez|first4=Dominic|last5=Lindner|first5=Garry|last6=Nadji|first6=Jonathan|last7=Bunn|first7=Stuart E.|bibcode=2018PLoSO..1397159A}}</ref> |
||
During extreme heat events, megabats like the [[little red flying fox]] (''Pteropus scapulatus'') must cool off and rehydrate by drinking from waterways, making them susceptible to opportunistic depredation by [[freshwater crocodiles]].<ref>{{cite AV media| url=https://www.youtube.com/watch?v=wi30w-Mk2yQ| date=10 April 2015|medium=video| publisher=BBC Earth|title=Flying Foxes Vs Freshwater Crocodile|access-date=22 May 2019}}</ref> |
During extreme heat events, megabats like the [[little red flying fox]] (''Pteropus scapulatus'') must cool off and rehydrate by drinking from waterways, making them susceptible to opportunistic depredation by [[freshwater crocodiles]].<ref>{{cite AV media| url=https://www.youtube.com/watch?v=wi30w-Mk2yQ| date=10 April 2015|medium=video| publisher=BBC Earth|title=Flying Foxes Vs Freshwater Crocodile|access-date=22 May 2019}}</ref> |
||
Megabats are the hosts of several parasite taxa. Known parasites include [[Nycteribiidae]] and [[Streblidae]] species ("bat flies").<ref>{{cite journal|doi=10.1186/s13071-017-2582-x|pmid=29284533|pmc=5747079|title=Hidden diversity of Nycteribiidae (Diptera) bat flies from the Malagasy region and insights on host-parasite interactions|journal=Parasites & Vectors|volume=10|issue=1|pages=630|year=2017|last1=Ramasindrazana|first1=Beza|last2=Goodman|first2=Steven M.|last3=Gomard|first3=Yann|last4=Dick|first4=Carl W.|last5=Tortosa|first5=Pablo}}</ref><ref>{{cite journal|doi=10.1186/s13071-018-2918-1|pmid=29859123|pmc=5984742|title=Rates of hematophagous ectoparasite consumption during grooming by an endemic Madagascar fruit bat|journal=Parasites & Vectors|volume=11|issue=1|pages=330|year=2018|last1=Ramanantsalama|first1=Riana V.|last2=Andrianarimisa|first2=Aristide|last3=Raselimanana|first3=Achille P.|last4=Goodman|first4=Steven M.}}</ref> |
|||
Blood parasites of the family [[Haemoproteidae]] also affect megabat species.<ref>{{cite journal|doi=10.1051/parasite/2012192137|pmid=22550624|pmc=3671437|title=The haemosporidian parasites of bats with description of ''Sprattiella'' alectogen. Nov., sp. Nov|journal=Parasite|volume=19|issue=2|pages=137–146|year=2012|last1=Landau|first1=I.|last2=Chavatte|first2=J.M.|last3=Karadjian|first3=G.|last4=Chabaud|first4=A.|last5=Beveridge|first5=I.}}</ref> |
|||
==Conservation== |
==Conservation== |
||
Revision as of 00:51, 23 May 2019
| Megabat | |
|---|---|

| |
| Little red flying foxes, Pteropus scapulatus | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Chiroptera |
| Superfamily: | Pteropodoidea |
| Family: | Pteropodidae Gray, 1821 |
| Subfamilies | |
| |

| |
| Distribution of megabats | |
| Synonyms | |
|
Pteropidae Gray, 1821[1] | |
Megabats constitute the family Pteropodidae of the order Chiroptera (bats). They are also called fruit bats, Old World fruit bats,[2] or, especially the genera Acerodon and Pteropus, flying foxes. The evolution of megabats has been determined primarily by genetic data, as the fossil record for this family is the worst of all bats. Megabats likely evolved in Australasia, with the common ancestor of all living pteropodids existing approximately 31 million years ago. Many megabat lineages likely originated in Melanesia, then dispersed to mainland Asia, the Mediterranean, and Africa over time. Megabats are found in tropical and subtropical areas of Eurasia, Africa, and Oceania.[3][4][5]
Compared to insectivorous bats, fruit bats are relatively large, and with some exceptions, do not navigate by echolocation. Most species are primarily frugivores and rely on their keen senses of sight and smell to locate food.[6] They reach sexual maturity slowly, and have a low reproductive output. Most species have one offspring at a time after a pregnancy of 4–6 months. This low reproductive output means that, after a population loss, their populations are slow to rebound, making them more susceptible to threats. A quarter of all megabat species are listed as threatened, with key responsible factors as habitat destruction and overhunting. Megabats are a popular food source in some areas, leading to population declines and extinction. While megabats can be a useful food resource, they are also of interest to public health as the natural reservoirs of several viruses than can affect humans.
Taxonomy
The family Pteropodidae was first described in 1821 by British zoologist John Edward Gray. Gray named the family "Pteropidae" (after the genus Pteropus) and placed it within the now-defunct order Fructivorae.[7] However, Gray's spelling was possibly based on a misunderstanding of the suffix of "Pteropus", and was subsequently changed to "Pteropodidae".[8] The Greek word pous of Pteropus is from the stem word pod-; therefore, Latinizing Pteropus correctly results in the prefix "Pteropod-". French biologist Charles Lucien Bonaparte was the first to use the corrected spelling Pteropodidae in 1838.[9] As of 2011, there were 186 species of megabat,[10] around a third of which are flying foxes of the genus Pteropus.[11]
In 1875, Irish zoologist George Edward Dobson was the first to split the order Chiroptera (bats) into two suborders: Megachiroptera (sometimes listed as Macrochiroptera) and Microchiroptera, which are commonly abbreviated to megabats and microbats.[12] Dobson selected these names to allude to the body size differences of these two groups, with many fruit-eating bats being larger than insect-eating bats. Pteropodidae was the only family he included within Megachiroptera.[8][12]
A 2001 study by Springer et al. found that the dichotomy of megabats and microbats did not accurately reflect their evolutionary lineages, however. Instead of Megachiroptera and Microchiroptera, they proposed the new suborders Yinpterochiroptera and Yangochiroptera. Yinpterochiroptera contained species formerly included in Megachiroptera (all of Pteropodidae), as well as several families formerly included in Microchiroptera: Megadermatidae, Rhinolophidae, Nycteridae, Craseonycteridae, and Rhinopomatidae.[13] Two superfamilies comprise Yinpterochiroptera: Rhinolophoidea—containing the above families formerly in Microchiroptera—and Pteropodoidea, which only contains Pteropodidae.[14]
In 1917, Danish mammalogist Knud Andersen divided Pteropodidae into three subfamilies: Macroglossinae, Pteropinae (corrected to Pteropodinae), and Harpyionycterinae.[15] However, a 1995 study found that Macroglossinae as previously defined (Eonycteris, Notopteris, Macroglossus, Syconycteris, Melonycteris, and Megaloglossus) was paraphyletic, meaning that the subfamily did not group all of the descendants of a common ancestor.[16] Subsequent publications consider Macroglossini as a tribe within Pteropodinae that contains only Macroglossus and Syconycteris.[17][10] Eonycteris and Melonycteris are within other tribes in Pteropodinae,[18][10] Megaloglossus was placed in Rousettinae:Myonycterini, and Notopteris is of uncertain placement.[10]
| |||
| Internal relationships of African Pteropodidae based on combined evidence of mitochondrial and nuclear DNA.[19] |
Other subfamilies and tribes within Pteropodidae have also undergone changes recently.[10] In 1997, Bergmans et al. classified the pteropodids into 6 subfamilies and 9 tribes based on their morphology, or physical characteristics.[10] A 2011 DNA study concluded that not all of these subfamilies were clades, or consisting of all the descendants of a common ancestor, and therefore they did not accurately depict the relationships between megabat species. Three of Bergmans's subfamilies received support: Cynopterinae, Harpyionycterinae, and Nyctimeninae. The three other clades recovered in this study consisted of Macroglossini, Epomophorinae + Rousettini, and Pteropodini + Melonycteris.[10] A 2016 DNA study focused only on African pteropodids (Harpyionycterinae, Rousettinae, and Epomophorinae) also challenged the 1997 Bergmans classification. All species formerly included in Epomophorinae were moved to Rousettinae, which was subdivided into additional tribes. The genus Eidolon, formerly in the tribe Rousettini of Rousettinae, was moved to its own subfamily, Eidolinae.[18] With these changes, the internal relationships of Pteropodidae are as follows:[10][18]
- Subfamily Pteropodinae
- Tribe Pteropodini
- Tribe Macroglossini
- Tribe Notopterini
- Subfamily Nyctimeninae
- Subfamily Harpyionyterinae (expanded to include Boneia)
- Subfamily Rousettinae (expanded)
- Tribe Rousettini (revised—now only includes Rousettus; formerly, Rousettini included Eidolon and Eonycteris)
- Tribe Eonycterini (new tribe)
- Tribe Scotonycterini
- Tribe Epomophorini
- Tribe Stenonycterini (new tribe)
- Tribe Myonycterini
- Tribe Plerotini
- Subfamily Cynopterinae
- Subfamily Eidolinae (new subfamily)
In 1984, Butler proposed an additional pteropodid subfamily, Propottininae, representing one extinct species described from a fossil discovered in Africa, Propotto leakeyi Simpson, 1967.[20] However, in 2018 the fossils were reexamined and determined to actually represent a lemur.[21]
Description

Megabats are so called for their larger weight and size, weighing up to one kilogram (two pounds) with wingspans reaching over one meter (several feet) in length. The tails are short if present, but are often effectively absent. The digestive system is structured to a herbivorous diet, sometimes restricted to soft fruit or nectar, and is shorter than those of the insectivorous microchiropterans. The tendency while resting amongst this group is to hang by the rear limb with the wings cloaking the body and its head facing forward.[22] The length of the digestive system is short for a herbivore, as the fibrous content is mostly separated by the action of the palate, tongue and teeth and then discarded. The ingested plant material is largely nectar, juices and pollen, the small seeds that are passed are often important to the dispersal of the tree species.[22]
Despite the fact that large body size is included as a defining characteristic of megabats, not all species of megabat are larger than microbats. Megabats range in size from 14.2 g (0.50 oz) in the spotted-winged fruit bat (Balionycteris maculata) to 1,075 g (37.9 oz) in the giant golden-crowned flying fox (Acerodon jubatus).[23] Megabats as a whole are often represented by the flying foxes of Pteropus and Acerodon; these species are outliers in their body size, leading some to overestimate the size of megabats.[8] One review showed that 28% of megabat species weigh less than 50 g (1.8 oz).[23]
Skeleton
Skull and dentition

The number of teeth a megabat has is dependent on the species, with a range of 24-34 teeth possible. All megabats have two or four each of upper and lower incisors, with the exception Bulmer's fruit bat (Aproteles bulmerae), which completely lacks incisors,[24] and the São Tomé collared fruit bat (Myonycteris brachycephala), which has two upper incisors and three lower incisors.[25] This makes it the only mammal species with an asymmetrical dental formula.[25]
All species have two upper and lower canine teeth. The number of premolars is variable, with four or six each of upper and lower premolars. The first upper and lower molars are always present, meaning that all megabats have at least four molars. The remaining molars may be present, present but reduced, or absent.[24] Megabat molars and premolars are simplified, with a reduction in the cusps and ridges resulting in a more flattened crown.[26]
Like most mammals, megabats are diphyodont, meaning that the young have a set of deciduous teeth (milk teeth) that falls out and is replaced by permanent teeth. For most species, there are 20 deciduous teeth. The deciduous set does not include molars.[26]
Postcrania

The scapulae of megabats are described as the most primitive of any chiropteran family. Within Pteropus, the scapula is narrow and has a large supraspinatous fossa. Additionally, the humerus is not as modified as in other families, lacking the same enlargement of the greater tuberosity and the pit at the anterior edge of the head. The shaft of the humerus is curved. While other bats only have claws on the thumbs of their forelimbs, most megabats possess a clawed second digit as well;[26] only Eonycteris, Dobsonia, Notopteris, and Neopteryx lack the second claw.[27] The first digit is the shortest, while the third digit is the longest. The second digit is incapable of flexion.[26]
Megabats' hindlimbs have all the same skeletal components as do humans, with the exception of an additional structure called the calcar, which is a cartilage spur arising from the calcaneus. The entire leg is rotated at the hip compared to normal mammal orientation, meaning that the knees face posteriorly. All five of the digits of the foot flex in the direction of the sagittal plane, with no digit capable of flexing in opposition (as seen in the feet of perching birds).[28]
Internal systems
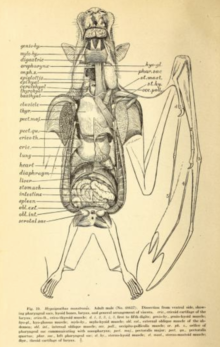
Flight is very energetically expensive, requiring several adaptations to the cardiovascular system. During flight, bats can raise their oxygen consumption by twenty times or more for sustained periods; top human athletes can reach a maximum of 15-20 times oxygen consumption for a few minutes at most.[29] Based on the straw-coloured fruit bat (Eidolon helvum) and hammer-headed bat (Hypsignathus monstrosus), megabats have a mean respiratory exchange ratio (carbon dioxide produced:oxygen used) of approximately 0.78. With the two above species as well as the gray-headed flying fox (Pteropus poliocephalus) and the Egyptian fruit bat (Rousettus aegyptiacus), maximum heart rates of the four species in flight varied between 476 beats per minute (P. poliocephalus) and 728 beats per minute (R. aegyptiacus). Maximum number of breaths per minute also varied, with P. poliocephalus with 163 breaths per minute and E. helvum with up to 316 breaths per minute.[30] Additionally, megabats have exceptionally large lung volumes relative to their sizes. While terrestrial mammals such as shrews have a lung volume of 0.03 cm3 per gram of body weight, species such as the Wahlberg's epauletted fruit bat (Epomophorus wahlbergi) have lung volumes 4.3 times greater at 0.13 cm3 of lung volume per gram of body weight.[29]
Megabats have rapid digestive systems, with a gut transit time of half an hour or less.[31] Many megabats have U-shaped stomachs. There is no distinct difference between the small and large intestine, nor a distinct beginning of the rectum. They have very high densities of intestinal microvilli, which creates a large surface area for the absorption of nutrients.[32]
Senses
Sight

With very few exceptions, megabats do not echolocate, and therefore rely on sight and smell to navigate.[33] They have large eyes that are positioned at the front of their heads.[34] Their eyes are larger than those of the common ancestor of all bats, with one study suggesting a trend of increasing eye size among pteropodids. A study that examined the eyes of 18 megabat species determined that the common blossom bat had the smallest eyes at a diameter of 5.03 mm (0.198 in), while the largest eyes were those of large flying fox at 12.34 mm (0.486 in) in diameter.[35] Megabat eyes are usually brown, though they can be red or orange as seen in some species of the following genera: Desmalopex, Mirimiri, Pteralopex, and some Pteropus.[36]
At high brightness levels, their visual acuity is poorer than humans'; at low brightness, however, their visual acuity exceeds humans'.[34] One study that examined the eyes of some Rousettus, Epomophorus, Eidolon, and Pteropus species determined that the former three genera possess a tapetum lucidum, while the Pteropus species do not. All species examined had retinae with both rod cells and cone cells, but only the Pteropus species had S-cones, which detect the shortest wavelengths of light (blue and/or ultraviolet); Pteropus bats are dichromatic, or possessing two kinds of cone cells. The other three genera, with their lack of S-cones, are monochromatic, or color blind. All genera had very high densities of rod cells, which corresponds with their nocturnal activity patterns, as increasing numbers of rod cells result in increased sensitivity to light. In Pteropus and Rousettus, measured rod cell densities were 350,000 – 800,000 per square millimeter, equivalent to or exceeding animals such as the house mouse, domestic cat, and domestic rabbit.[33]
Smell
Megabats use smell to find food sources such as fruit and nectar.[37] They have keen senses of smell that rival those of the domestic dog.[38] Tube-nosed fruit bats such as the eastern tube-nosed bat (Nyctimene robinsoni) have stereo olfaction, meaning that they are able to map and follow odor plumes three-dimensionally.[39] Along with most (or perhaps all) other bat species, megabats also use scent for mothers and offspring to recognize each other, as well as for recognition of individuals.[37] In flying foxes, males have enlarged androgen-sensitive sebaceous glands on their shoulders that they use for scent-marking their territories, particularly during the mating season. The secretions of these glands vary by species—of the 65 chemical compounds isolated from the glands of four species, no compound was found in all species.[40] Males also engage in "urine washing", meaning that they coat themselves in their own urine.[40][41]
Taste
Megabats possess the TAS1R2 gene, meaning that they have the ability to detect sweetness in foods. This gene is present among all bats except vampire bats. Like all bats, megabats cannot taste umami, based on the absence of the TAS1R1 gene. Outside of bats, only giant pandas have been shown to also lack this gene.[37] Megabats also possess multiple TAS2R genes, indicating an ability to taste bitterness.[42]
Evolution
Fossil record and divergence times
The fossil record for pteropodid bats is the most incomplete of any bat family. Several factors could explain why so few pteropodid fossils have been discovered: tropical regions where their fossils might be found are undersampled relative to Europe and North America; conditions for fossilization are poor in the tropics, which could lead to fewer fossils overall; and fossils may have been created, but they may have been destroyed by subsequent geological activity.[43] It is estimated that more than 98% of the pteropodid fossil record is missing.[44] However, even without fossils, the age and divergence times of the family can still be estimated by using computational phylogenetics. Pteropodidae split from the superfamily Rhinolophoidea (which contains all the other families of the suborder Yinpterochiroptera) approximately 58 Mya (million years ago).[44] The ancestor of the crown group of Pteropodidae, or all living species, lived approximately 31 Mya.[45]
Biogeography

The family Pteropodidae likely originated in Australasia.[18] The Melanesian Islands, including New Guinea, are a plausible candidate for the origin of most megabat subfamilies, with the exception of Cynopterinae;[18] the cynopterines likely originated on the Sunda Shelf.[45] From these regions, pteropodids were able to colonize other areas, including continental Asia and Africa. Megabats reached Africa in at least four distinct events. The four proposed events are represented by (1) Scotonycteris, (2) Rousettus, (3) Scotonycterini, and (4) the "endemic Africa clade", which includes Stenonycterini, Plerotini, Myonycterini, and Epomophorini as proposed by Almeida et al. 2016. It is unknown when megabats reached Africa, but several tribes (Scotonycterini, Stenonycterini, Plerotini, Myonycterini, and Epomophorini) were present by the Late Miocene. How megabats reached Africa is also unknown. It has been proposed that they could have arrived via the Middle East before it became more arid at the end of the Miocene. Conversely, they could have reached the continent via the Gomphotherium land bridge, which connected Africa/the Arabian Peninsula to Eurasia. The genus Pteropus (flying foxes), which is not found on mainland Africa, is proposed to have dispersed from Melanesia via island hopping across the Indian Ocean,[46] though this is less likely for other megabat genera, which have smaller body size and thus have more limited flight capabilities.[18]
Loss of echolocation
Megabats make up the only family (Pteropodidae) in order Chiroptera that is not capable of laryngeal echolocation. Echolocation and flight evolved early in the lineage of chiropterans. Although echolocation was later lost in family Pteropodidae,[47] bats in the genus Rousettus are capable of primitive echolocation through clicking their tongues,[48] and some species have been shown to create clicks similar to those of echolocating bats using their wings.[49][50]
Both echolocation and flight are energetically expensive processes.[51] The nature of the flight and echolocation mechanism of bats allows for creation of echolocation pulses with minimal energy use.[52] Energetic coupling of these two processes is thought to have allowed for both energetically expensive processes to evolve in bats. The loss of echolocation may be due to the uncoupling of flight and echolocation in megabats.[53] The larger average body size of megabats compared to echolocating bats[54] suggests that a larger body size disrupts the flight-echolocation coupling and made echolocation too energetically expensive to be conserved in megabats.[53]
Biology and ecology
Reproduction and life cycle

Relative to their sizes, megabats have low reproductive outputs and delayed sexual maturity, with females of most species not giving birth until the age of one or two.[55]: 6 Some megabats appear to be able to breed throughout the year, but the majority of species are likely seasonal breeders.[27] Gestation length is variable. The Fischer's pygmy fruit bat (Haplonycteris fischeri) has the longest gestation length of any bat species, taking up to 11.5 months to carry a pregnancy to term.[56] Shorter gestation lengths are found in the greater short-nosed fruit bat (Cynopterus sphinx) with a period of three months.[57] Among all species, a gestation length of 4-6 months is common. Some species such as the straw-coloured fruit bat have the reproductive adaptation of delayed implantation, meaning that copulation occurs in June or July, but the zygote does not implant into the uterine wall until months later in November. The Fischer's pygmy fruit bat has the adaptation of post-implantation delay, meaning that development of the embryo is suspended for up to eight months after implantation in the uterine wall. This is responsible for its very long pregnancies. The litter size of all megabats is usually one offspring.[55]: 6 Megabats, like all bats, are long-lived relative to their size. Some captive megabats have had lifespans exceeding thirty years.[27]
Diet and foraging
Most megabats are primarily frugivorous, meaning that they mostly consume fruit.[58] Throughout the family, a diverse array of fruit is consumed from nearly 188 plant genera.[59] Some species are also nectarivorous, meaning that they also drink nectar from flowers.[58] Other food resources include leaves, shoots, buds, flowers, pollen, seed pods, sap, cones, bark, and twigs.[60] They are prodigious eaters, and can consume up to 2.5 times their own body weight in fruit per night.[59]
Predators and parasites
Megabats have few native predators, especially those living on islands: species like the small flying fox (Pteropus hypomelanus) have no known natural predators.[61] Non-native predators of flying foxes include domestic cats and rats. The mangrove monitor, which is a native predator for some megabat species but an introduced predator for others, opportunistically preys on megabats, as it is a capable tree climber.[62] Another invasive species, the brown tree snake can seriously impact megabat populations; the brown tree snake consumes so many offspring that it reduced the recruitment of the Guam population of the Mariana fruit bat (Pteropus mariannus) to essentially zero.[63] Native predators of megabats include crocodilians. The saltwater crocodile, a native species, is a known predator of megabats, based on analysis of crocodile stomach contents in northern Australia.[64] During extreme heat events, megabats like the little red flying fox (Pteropus scapulatus) must cool off and rehydrate by drinking from waterways, making them susceptible to opportunistic depredation by freshwater crocodiles.[65]
Megabats are the hosts of several parasite taxa. Known parasites include Nycteribiidae and Streblidae species ("bat flies").[66][67] Blood parasites of the family Haemoproteidae also affect megabat species.[68]
Conservation
Status

As of 2014, the IUCN evaluated a quarter of all megabat species as threatened with endangerment, which includes species listed as critically endangered, endangered, and vulnerable. Megabats are substantially threatened by humans, as they are hunted for food and medicinal uses. Additionally, they are culled for actual or perceived damage to agriculture, especially to fruit production.[70] As of 2019, the IUCN has evaluations for 187 megabat species. The status breakdown is as follows:[71]
- Extinct: 4 species (2.1%)
- Critically endangered: 8 species (4.3%)
- Endangered: 16 species (8.6%)
- Vulnerable: 37 species (19.8%)
- Near-threatened: 13 species (7.0%)
- Least-concern: 89 species (47.6%)
- Data deficient: 20 species (10.7%)
Factors causing decline
Anthropogenic sources

Megabats are threatened by habitat destruction by humans, which takes several forms. Deforestation of their habitats has resulted in the loss of critical roosting habitat. Deforestation also results in the loss of food resource, as native fruit-bearing trees are felled. Habitat loss and resulting urbanization leads to construction of new roadways, making megabat colonies easier to access for overharvesting. Additionally, habitat loss via deforestation compounds natural threats, as fragmented forests are more susceptible to damage from typhoon-force winds.[55]: 7 Cave-roosting megabats are threatened by human disturbance at their roost sites. Guano mining is a livelihood in some countries within their range, bringing people to caves. Caves are also disturbed via mineral mining and cave tourism.[55]: 8
Megabats are also intentionally killed by humans for several reasons. A large source of mortality is by human persecution stemming from agricultural conflicts. Though some megabats have been documented to have a preference for native fruit trees over fruit crops, deforestation can reduce the megabat food supply, causing them to rely on fruit crops.[55]: 8 They are shot, beaten to death, or poisoned to reduce their populations. Mortality also occurs via accidental entanglement into netting used to prevent the bats from eating fruit.[72] Culling can dramatically reduce megabat populations. In Mauritius, over 40,000 Mauritian flying foxes were culled in a two-year period, reducing its population by an estimated 45%.[73] Megabats are also killed by electrocution. In one Australian orchard, it is estimated that over 21,000 bats were electrocuted to death in an 8-week period.[74] Farmers construct electrified grids over their fruit trees to kill megabats before they can consume their crop. The grids are questionably effective at preventing crop loss, with one farmer who operated such a grid estimating that they still lost 100–120 tonnes (220,000–260,000 lb) of fruit to flying foxes in a year.[75] Some electrocution deaths are also accidental, such as when bats fly into overhead power lines.[76]
Climate change causes flying fox mortality and a source of concern for species persistence. Extreme heat waves in Australia have been responsible for the deaths of more than 30,000 flying foxes from 1994 to 2008. Females and young bats are most susceptible to extreme heat, which affects a population's ability to recover.[77] Megabats are threatened by sea level rise associated with climate change, as several species are endemic to low-lying atolls.[78]
Natural sources

Because many species are endemic to a single island, they are vulnerable to random events such as typhoons. A 1979 typhoon halved the remaining population of the Rodrigues flying fox. Typhoons result in indirect mortality as well: Because they defoliate the trees, megabats are more visible and easily hunted by humans. Food resources for the bats become scarce after major storms, and megabats resort to riskier foraging strategies such as consuming fallen fruit off the ground. There, they are more vulnerable to depredation by domestic cats, dogs, and pigs.[69] As many megabat species are located in the tectonically active Ring of Fire, they are also threatened by volcanic eruptions. Flying foxes have been nearly exterminated from the island of Anatahan following a series of eruptions beginning in 2003.[79]
Relationship to people
Food
Megabats are killed and consumed as bushmeat throughout their range. Bats are consumed extensively throughout Asia, as well as in islands of the West Indian Ocean and the Pacific, where Pteropus species are heavily hunted. In continental Africa, where no Pteropus species live, its largest megabat, the straw-coloured fruit bat, is a preferred hunting target.[80]
In Guam, consumption of the Mariana fruit bat exposes locals to the neurotoxin beta-Methylamino-L-alanine (BMAA) which may later lead to neurodegenerative diseases. BMAA may become biomagnified in humans who consume flying foxes; flying foxes are exposed to BMAA by eating cycad fruits.[81][82][83]
As disease reservoirs


Megabats are the reservoirs of several viruses that can affect humans and cause disease. They can carry filoviruses, including the Ebola virus (EBOV) and Marburgvirus. Species that have tested positive for the presence of EBOV include Franquet's epauletted fruit bat, the hammer-headed fruit bat, and the little collared fruit bat. Additionally, antibodies against EBOV have been found in the straw-coloured fruit bat, Gambian epauletted fruit bat, Peters's dwarf epauletted fruit bat, Veldkamp's dwarf epauletted fruit bat, Leschenault's rousette, and the Egyptian fruit bat. Marburgvirus has been confirmed in one species, the Egyptian fruit bat.[84][85][86]
Other megabats implicated as disease reservoirs are primarily Pteropus species. Notably, flying foxes can transmit lyssaviruses, which cause rabies. In Australia the rabies virus is not naturally present; Australian bat lyssavirus is the only lyssavirus present. Australian bat lyssavirus was first identified in 1996; it is very rarely transmitted to humans. Transmission occurs from the bite or scratch of an infected animal, but can also occur from getting the infected animal's saliva in a mucous membrane or an open wound. Exposure to flying fox blood, urine, or feces is not a risk of exposure to Australian bat lyssavirus. Since 1994, there have been three records of people getting infected with it—all three were in Queensland and each case was fatal.[87]
Flying foxes are also reservoirs of henipaviruses such as Hendra virus and Nipah virus. Hendra virus was first identified in 1994; it also rarely occurs humans. From 1994 to 2013, there have been seven reported cases of Hendra virus affecting people, four of which were fatal. The hypothesized primary route of human infection is via contact with horses that have come into contact with flying fox urine.[88] There are no documented instances of direct transmission between flying foxes and humans.[89] As of 2012, there is a vaccine available for horses to decrease the likelihood of infection and transmission.[90]
Nipah virus was first identified in 1998 in Malaysia. Since 1998, there have been several Nipah outbreaks in Malaysia, Singapore, India, and Bangladesh, resulting in over 100 casualties. A 2018 outbreak in Kerala, India resulted in 19 humans infected, of which 17 died.[91] The overall fatality rate is 40-75%. Humans can contract Nipah virus from direct contact with flying foxes or their fluids, through exposure to an intermediate host such as domestic pigs, or from contact with an infected person.[92] A 2014 study of the Indian flying fox and Nipah virus found that while Nipah virus outbreaks are more likely in areas preferred by flying foxes, "the presence of bats in and of itself is not considered a risk factor for Nipah virus infection." Rather, the consumption of date palm sap is a significant route of transmission. The practice of date palm sap collection involves placing collecting pots at date palm trees. Indian flying foxes have been observed licking the sap as it flows into the pots, as well as defecating and urinating in proximity to the pots. In this way, humans who drink the palm sap can be exposed to the bats' viruses. The use of bamboo skirts on collecting pots lowers the risk of contamination from bat fluids.[93]
Flying foxes can transmit several non-lethal diseases as well, such as Menangle virus[94] and Nelson Bay virus.[95] These viruses rarely affect humans and few cases have been reported.[94][95] While other bat species have been suspected or implicated as the reservoir of diseases such as SARS and Ebola, flying foxes are not suspected as hosts for either causative virus.[96]
List of genera




The family Pteropodidae is divided into seven subfamilies represented by 44–46 genera:
Family Pteropodidae
- subfamily Cynopterinae
- genus Aethalops – pygmy fruit bats
- genus Alionycteris
- genus Balionycteris
- genus Chironax
- genus Cynopterus – dog-faced fruit bats or short-nosed fruit bats
- genus Dyacopterus – Dayak fruit bats
- genus Haplonycteris
- genus Latidens
- genus Megaerops
- genus Otopteropus
- genus Penthetor
- genus Ptenochirus – musky fruit bats
- genus Sphaerias
- genus Thoopterus
- subfamily Eidoloninae
- genus Eidolon – straw-coloured fruit bats
- subfamily Harpiyonycterinae
- genus Aproteles
- genus Dobsonia – naked-backed fruit bats
- genus Harpyionycteris
- genus Boneia
- subfamily Nyctimeninae
- genus Nyctimene – tube-nosed fruit bats
- genus Paranyctimene
- subfamily Pteropodinae
- genus Melonycteris
- genus Acerodon
- genus Desmalopex
- genus Mirimiri
- genus Neopteryx
- genus Pteralopex
- genus Pteropus – flying foxes
- genus Styloctenium
- genus Macroglossus – long-tongued fruit bats
- genus Syconycteris – blossom bats
- subfamily Rousettinae
- genus Rousettus – rousette fruit bats
- genus Eonycteris – dawn fruit bats
- genus Casinycteris
- genus Scotonycteris
- genus Epomophorus – epauletted fruit bats
- genus Epomops – epauletted bats
- genus Hypsignathus
- genus Nanonycteris
- genus Micropteropus – dwarf epauletted bats
- genus Stenonycteris
- genus Myonycteris – little collared fruit bats
- genus Lissonycteris
- genus Megaloglossus
- genus Plerotes
- genus Notopteris – long-tailed fruit bats
References
- ^ a b McKenna, M. C.; Bell, S. K. (1997). Classification of mammals: above the species level. Columbia University Press. p. 296. ISBN 9780231528535.
- ^ Mickleburgh, Simon P.; Hutson, Anthony M.; Racey, Paul A. "Old World Fruit Bats: Introduction". International Union for Conservation of Nature. Archived from the original on 17 March 2014. Retrieved 19 July 2013.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Our European Bats". BatLife Europe. Retrieved 24 April 2017.
- ^ Luzynski, Kenneth Cody; Sluzas, Emily Margaret; Wallen, Megan Marie. "Pteropodidae: Old World fruit bats". Animal Diversity Web/University of Michigan.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Smith, Charles H. "PTEROPODIDAE (Fruit Bats/Flying Foxes)". MAMMFAUN. Western Kentucky University.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) (includes range map) - ^ Neuweiler, Gerhard (2000). The Biology of Bats. Oxford University Press. ISBN 978-0-19-509950-8. Retrieved 28 March 2015.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gray, J. E. (1821). "On the natural arrangement of vertebrose animals". London Medical Repository (25): 296–310.
- ^ a b c Hutcheon, J. M.; Kirsch, J. A. (2006). "A moveable face: deconstructing the Microchiroptera and a new classification of extant bats". Acta Chiropterologica. 8 (1): 1–10. doi:10.3161/1733-5329(2006)8[1:AMFDTM]2.0.CO;2.
- ^ Jackson, S.; Jackson, S. M.; Groves, C. (2015). Taxonomy of Australian Mammals. Csiro Publishing. p. 230. ISBN 9781486300136.
- ^ a b c d e f g h Almeida, Francisca C.; Giannini, Norberto P.; Desalle, Rob; Simmons, Nancy B. (2011). "Evolutionary relationships of the old world fruit bats (Chiroptera, Pteropodidae): Another star phylogeny?". BMC Evolutionary Biology. 11: 281. doi:10.1186/1471-2148-11-281. PMC 3199269. PMID 21961908.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Taxonomy=Pteropus". IUCN Red List of Threatened Species. 2019. Retrieved 19 May 2019.
- ^ a b Dobson, G. E. (1875). "Conspectus of the suborders, families, and genera of Chiroptera arranged according to their natural affinities". The Annals and Magazine of Natural History; Zoology, Botany, and Geology. 4. 16 (95).
- ^ Springer, M. S.; Teeling, E. C.; Madsen, O.; Stanhope, M. J.; De Jong, W. W. (2001). "Integrated fossil and molecular data reconstruct bat echolocation". Proceedings of the National Academy of Sciences. 98 (11): 6241–6246. Bibcode:2001PNAS...98.6241S. doi:10.1073/pnas.111551998. PMC 33452. PMID 11353869.
- ^ Ungar, P. (2010). Mammal Teeth: Origin, Evolution, and Diversity. JHU Press. p. 166. ISBN 9780801899515.
- ^ Giannini, Norberto P.; Simmons, Nancy B. (2003). "A phylogeny of megachiropteran bats (Mammalia: Chiroptera: Pteropodidae) based on direct optimization analysis of one nuclear and four mitochondrial genes". Cladistics. 19 (6): 496–511. doi:10.1111/j.1096-0031.2003.tb00385.x.
- ^ Colgan, D. J.; Flannery, T. F. (1995). "A Phylogeny of Indo-West Pacific Megachiroptera Based on Ribosomal DNA". Systematic Biology. 44 (2): 209–220. doi:10.1093/sysbio/44.2.209.
- ^ Bergmans, W. (1997). "Taxonomy and biogeography of African fruit bats (Mammalia, Megachiroptera). 5. The genera Lissonycteris Andersen, 1912, Myonycteris Matschie, 1899 and Megaloglossus Pagenstecher, 1885; general remarks and conclusions; annex: key to all species". Beaufortia. 47 (2): 11–90.
- ^ a b c d e f Almeida, Francisca; Giannini, Norberto Pedro; Simmons, Nancy B. (2016). "The Evolutionary History of the African Fruit Bats (Chiroptera: Pteropodidae)". Acta Chiropterologica. 18: 73–90. doi:10.3161/15081109ACC2016.18.1.003.
- ^ Cunhaalmeida, Francisca; Giannini, Norberto Pedro; Simmons, Nancy B. (2016). "The Evolutionary History of the African Fruit Bats (Chiroptera: Pteropodidae)". Acta Chiropterologica. 18: 73–90. doi:10.3161/15081109ACC2016.18.1.003.
- ^ Butler, P. M. (1984). "Macroscelidea, Insectivora and Chiroptera from the Miocene of east Africa". Palaeovertebrata. 14 (3): 117–198.
- ^ Gunnell, Gregg F.; Boyer, Doug M.; Friscia, Anthony R.; Heritage, Steven; Manthi, Fredrick Kyalo; Miller, Ellen R.; Sallam, Hesham M.; Simmons, Nancy B.; Stevens, Nancy J.; Seiffert, Erik R. (2018). "Fossil lemurs from Egypt and Kenya suggest an African origin for Madagascar's aye-aye". Nature Communications. 9 (1): 3193. Bibcode:2018NatCo...9.3193G. doi:10.1038/s41467-018-05648-w. PMC 6104046. PMID 30131571.
- ^ a b Richards, G.C. (1983). "Fruit-bats and their relatives". In Strahan, R. (ed.). Complete book of Australian mammals. The national photographic index of Australian wildlife (1 ed.). London: Angus & Robertson. pp. 271–273. ISBN 978-0207144547.
- ^ a b Hutcheon, James M.; Garland Jr, Theodore (2004). "Are Megabats Big?". Journal of Mammalian Evolution. 11 (3/4): 257–277. doi:10.1023/B:JOMM.0000047340.25620.89.
- ^ a b Giannini, N. P.; Simmons, N. B. (2007). "Element homology and the evolution of dental formulae in megachiropteran bats (Mammalia: Chiroptera: Pteropodidae)" (PDF). American Museum Novitates. 3559: 1–27. doi:10.1206/0003-0082(2007)3559[1:EHATEO]2.0.CO;2.
- ^ a b Juste, J.; Ibáñez, C. (1993). "An asymmetric dental formula in a mammal, the Sao Tomé Island fruit bat Myonycteris brachycephala (Mammalia: Megachiroptera)". Canadian Journal of Zoology. 71 (1): 221–224. doi:10.1139/z93-030.
- ^ a b c d Vaughan, Terry (1970). "Chapter 3: The Skeletal System". In Wimsatt, W. (ed.). Biology of Bats. Academic Press. pp. 103–136. ISBN 9780323151191.
- ^ a b c Nowak, Ronald M.; Pillsbury Walker, Ernest (1999). Walker's Mammals of the World. Vol. Volume 1. JHU Press. p. 258. ISBN 9780801857898.
{{cite book}}:|volume=has extra text (help) - ^ Bennett, M. B. (1993). "Structural modifications involved in the fore- and hind limb grip of some flying foxes (Chiroptera: Pteropodidae)". Journal of Zoology. 229 (2): 237–248. doi:10.1111/j.1469-7998.1993.tb02633.x.
- ^ a b Maina, J. N.; King, A. S. (1984). "Correlations between structure and function in the design of the bat lung: a morphometric study" (PDF). Journal of Experimental Biology. 11: 43–61.
- ^ Carpenter, Roger E. (1986). "Flight Physiology of Intermediate-Sized Fruit Bats (Pteropodidae)" (PDF). Journal of Experimental Biology. 120: 79–103.
- ^ Nelson, John E. Fauna of Australia (PDF) (Report). Vol. 1B. Australian Government Department of the Environment and Energy.
- ^ Schmidt-Rhaesa, Andreas, ed. (2017). Comparative Anatomy of the Gastrointestinal Tract in Eutheria II. Walter de Gruyter GmbH & Co KG. ISBN 9783110560671.
- ^ a b Müller, Brigitte; Goodman, Steven M.; Peichl, Leo (2007). "Cone Photoreceptor Diversity in the Retinas of Fruit Bats (Megachiroptera)". Brain, Behavior and Evolution. 70 (2): 90–104. doi:10.1159/000102971. PMID 17522478.
- ^ a b Graydon, M.; Giorgi, P.; Pettigrew, J. (1987). "Vision in Flying-Foxes (Chiroptera:Pteropodidae)". Journal of the Australian Mammal Society. 10 (2): 101–105.
- ^ Thiagavel, Jeneni; Cechetto, Clément; Santana, Sharlene E.; Jakobsen, Lasse; Warrant, Eric J.; Ratcliffe, John M. (2018). "Auditory opportunity and visual constraint enabled the evolution of echolocation in bats". Nature Communications. 9 (1): 98. Bibcode:2018NatCo...9...98T. doi:10.1038/s41467-017-02532-x. PMC 5758785. PMID 29311648.
- ^ Giannini, Norberto P.; Almeida, Francisca Cunha; Simmons, Nancy B.; Helgen, Kristofer M. (2008). "The systematic position of Pteropus leucopterus and its bearing on the monophyly and relationships of Pteropus (Chiroptera: Pteropodidae)". Acta Chiropterologica. 10: 11–20. doi:10.3161/150811008X331054.
- ^ a b c Jones, Gareth; Teeling, Emma C.; Rossiter, Stephen J. (2013). "From the ultrasonic to the infrared: Molecular evolution and the sensory biology of bats". Frontiers in Physiology. 4: 117. doi:10.3389/fphys.2013.00117. PMC 3667242. PMID 23755015.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Schwab, I. R. (2005). "A choroidal sleight of hand". British Journal of Ophthalmology. 89 (11): 1398. doi:10.1136/bjo.2005.077966. PMC 1772916. PMID 16267906.
- ^ Schwab, I. R.; Pettigrew, J. (2005). "A choroidal sleight of hand". British Journal of Ophthalmology. 89 (11): 1398. doi:10.1136/bjo.2005.077966. PMC 1772916. PMID 16267906.
- ^ a b Wood, W. F.; Walsh, A.; Seyjagat, J.; Weldon, P. J. (2005). "Volatile Compounds in Shoulder Gland Secretions of Male Flying Foxes, Genus Pteropus (Pteropodidae, Chiroptera)". Z Naturforsch C. 60 (9–10): 779–784. doi:10.1515/znc-2005-9-1019.
- ^ Wagner, J. (2008). "Glandular secretions of male Pteropus (Flying foxes): preliminary chemical comparisons among species". Independent Study Project (Isp) Collection. Independent Study Project (ISP) Collection.
- ^ Li, Diyan; Zhang, Jianzhi (2014). "Diet Shapes the Evolution of the Vertebrate Bitter Taste Receptor Gene Repertoire". Molecular Biology and Evolution. 31 (2): 303–309. doi:10.1093/molbev/mst219. PMC 3907052. PMID 24202612.
- ^ Eiting, Thomas P.; Gunnell, Gregg F. (2009). "Global Completeness of the Bat Fossil Record". Journal of Mammalian Evolution. 16 (3): 151–173. doi:10.1007/s10914-009-9118-x.
- ^ a b Teeling, E. C.; Springer, M. S.; Madsen, O.; Bates, P.; O'Brien, S. J.; Murphy, W. J. (2005). "A Molecular Phylogeny for Bats Illuminates Biogeography and the Fossil Record" (PDF). Science. 307 (5709): 580–584. Bibcode:2005Sci...307..580T. doi:10.1126/science.1105113. PMID 15681385.
- ^ a b Almeida, Francisca C.; Giannini, Norberto P.; Desalle, Rob; Simmons, Nancy B. (2009). "The phylogenetic relationships of cynopterine fruit bats (Chiroptera: Pteropodidae: Cynopterinae)". Molecular Phylogenetics and Evolution. 53 (3): 772–783. doi:10.1016/j.ympev.2009.07.035. PMID 19660560.
- ^ o'Brien, John; Mariani, Carol; Olson, Link; Russell, Amy L.; Say, Ludovic; Yoder, Anne D.; Hayden, Tom J. (2009). "Multiple colonisations of the western Indian Ocean by Pteropus fruit bats (Megachiroptera: Pteropodidae): The furthest islands were colonised first". Molecular Phylogenetics and Evolution. 51 (2): 294–303. doi:10.1016/j.ympev.2009.02.010. PMID 19249376.
- ^ Springer MS, Teeling EC, Madsen O, Stanhope MJ, de Jong WW (May 2001). "Integrated fossil and molecular data reconstruct bat echolocation". Proceedings of the National Academy of Sciences of the United States of America. 98 (11): 6241–6. Bibcode:2001PNAS...98.6241S. doi:10.1073/pnas.111551998. PMC 33452. PMID 11353869.
- ^ Holland RA, Waters DA, Rayner JM (December 2004). "Echolocation signal structure in the Megachiropteran bat Rousettus aegyptiacus Geoffroy 1810". The Journal of Experimental Biology. 207 (Pt 25): 4361–9. doi:10.1242/jeb.01288. PMID 15557022.
- ^ Boonman A, Bumrungsri S, Yovel Y (December 2014). "Nonecholocating fruit bats produce biosonar clicks with their wings". Current Biology. 24 (24): 2962–7. Bibcode:1996CBio....6.1213A. doi:10.1016/j.cub.2014.10.077. PMID 25484290.
- ^ Ravindran, Sandeep (4 December 2014). "When It Comes to Echolocation, Some Bats Just Wing It". National Geographic.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Speakman JR, Racey PA (April 1991). "No cost of echolocation for bats in flight". Nature. 350 (6317): 421–3. Bibcode:1991Natur.350..421S. doi:10.1038/350421a0. PMID 2011191.
- ^ Lancaster WC, Henson OW, Keating AW (January 1995). "Respiratory muscle activity in relation to vocalization in flying bats". The Journal of Experimental Biology. 198 (Pt 1): 175–91. PMID 7891034.
- ^ a b Altringham, J.D. (2011). Echolocation and other senses. New York: Oxford University Press.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hutcheon, J.M.; Garland, T.J. (1995). "Are megabats big?". Journal of Mammalian Evolution. 11 (3/4): 257–277. doi:10.1023/B:JOMM.0000047340.25620.89.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d e Mickleburgh, S. P.; Hutson, A. M.; Racey, P. A. (1992). Old World fruit bats: An action plan for their conservation (PDF) (Report). Gland, Switzerland: IUCN.
- ^ Heideman, Paul D. (1988). "The timing of reproduction in the fruit bat Haplonycteris fischeri(Pteropodidae): Geographic variation and delayed development". Journal of Zoology. 215 (4): 577–595. doi:10.1111/j.1469-7998.1988.tb02396.x.
- ^ Nowak, Ronald M.; Pillsbury Walker, Ernest (1999). Walker's Mammals of the World. Vol. Volume 1. JHU Press. p. 287. ISBN 9780801857898.
{{cite book}}:|volume=has extra text (help) - ^ a b Dumont, Elizabeth R.; O'Neal, Reilly (2004). "Food Hardness and Feeding Behavior in Old World Fruit Bats (Pteropodidae)". Journal of Mammalogy. 85: 8–14. doi:10.1644/BOS-107.
- ^ a b Yin, Qiuyuan; Zhu, Lei; Liu, Di; Irwin, David M.; Zhang, Shuyi; Pan, Yi-Hsuan (2016). "Molecular Evolution of the Nuclear Factor (Erythroid-Derived 2)-Like 2 Gene Nrf2 in Old World Fruit Bats (Chiroptera: Pteropodidae)". PLOS ONE. 11 (1): e0146274. Bibcode:2016PLoSO..1146274Y. doi:10.1371/journal.pone.0146274. PMC 4703304. PMID 26735303.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Courts, S. E. (1998). "Dietary strategies of Old World Fruit Bats (Megachiroptera, Pteropodidae): How do they obtain sufficient protein?". Mammal Review. 28 (4): 185–194. doi:10.1046/j.1365-2907.1998.00033.x.
- ^ Reeder, Deeann M.; Kunz, Thomas H.; Widmaier, Eric P. (2004). "Baseline and stress-induced glucocorticoids during reproduction in the variable flying fox,Pteropus hypomelanus (Chiroptera: Pteropodidae)". Journal of Experimental Zoology. 301A (8): 682–690. doi:10.1002/jez.a.58. PMID 15286948.
- ^ Buden, Don; Helgen, Kristofer M.; Wiles, Gary (2013). "Taxonomy, distribution, and natural history of flying foxes (Chiroptera, Pteropodidae) in the Mortlock Islands and Chuuk State, Caroline Islands". ZooKeys (345): 97–135. doi:10.3897/zookeys.345.5840. PMC 3817444. PMID 24194666.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Esselstyn, Jacob A; Amar, Arjun; Janeke, Dustin (2006). "Impact of Posttyphoon Hunting on Mariana Fruit Bats (Pteropus mariannus)". Pacific Science. 60 (4): 531. doi:10.1353/psc.2006.0027.
- ^ Adame, Maria Fernanda; Jardine, Timothy D.; Fry, Brian; Valdez, Dominic; Lindner, Garry; Nadji, Jonathan; Bunn, Stuart E. (2018). "Estuarine crocodiles in a tropical coastal floodplain obtain nutrition from terrestrial prey". PLOS ONE. 13 (6): e0197159. Bibcode:2018PLoSO..1397159A. doi:10.1371/journal.pone.0197159. PMC 5991389. PMID 29874276.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Flying Foxes Vs Freshwater Crocodile (video). BBC Earth. 10 April 2015. Retrieved 22 May 2019.
- ^ Ramasindrazana, Beza; Goodman, Steven M.; Gomard, Yann; Dick, Carl W.; Tortosa, Pablo (2017). "Hidden diversity of Nycteribiidae (Diptera) bat flies from the Malagasy region and insights on host-parasite interactions". Parasites & Vectors. 10 (1): 630. doi:10.1186/s13071-017-2582-x. PMC 5747079. PMID 29284533.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ramanantsalama, Riana V.; Andrianarimisa, Aristide; Raselimanana, Achille P.; Goodman, Steven M. (2018). "Rates of hematophagous ectoparasite consumption during grooming by an endemic Madagascar fruit bat". Parasites & Vectors. 11 (1): 330. doi:10.1186/s13071-018-2918-1. PMC 5984742. PMID 29859123.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Landau, I.; Chavatte, J.M.; Karadjian, G.; Chabaud, A.; Beveridge, I. (2012). "The haemosporidian parasites of bats with description of Sprattiella alectogen. Nov., sp. Nov". Parasite. 19 (2): 137–146. doi:10.1051/parasite/2012192137. PMC 3671437. PMID 22550624.
- ^ a b Pierson, E. D.; Rainey, W. E. (1992). "The biology of flying foxes of the genus Pteropus: a review" (PDF). Biological Report. 90 (23).
- ^ Aziz, S.A.; Olival, K.J.; Bumrungsri, S.; Richards, G.C.; Racey, P.A. (2016). "The Conflict Between Pteropodid Bats and Fruit Growers: Species, Legislation and Mitigation". In Voigt, C.; Kingston, T. (eds.). Bats in the Anthropocene: Conservation of Bats in a Changing World. Springer. ISBN 978-3-319-25220-9.
- ^ "Taxonomy=Pteropodidae". IUCN Red List of Threatened Species. 2019. Retrieved 19 May 2019.
- ^ Vincenot, Christian Ernest; Koyama, Lina; Russo, Danilo (2015). "Near threatened? First report of unsuspected human-driven decline factors in the Ryukyu flying fox (Pteropus dasymallus) in Japan". Mammalian Biology - Zeitschrift für Säugetierkunde. 80 (4): 273. doi:10.1016/j.mambio.2015.03.003.
- ^ Vincenot, Christian E; Florens, F. B. Vincent; Kingston, Tigga (2017). "Can we protect island flying foxes?". Science. 355 (6332): 1368. Bibcode:2017Sci...355.1368V. doi:10.1126/science.aam7582. PMID 28360279.
- ^ McIlwee, A. P; Martin, L (2002). "On the intrinsic capacity for increase of Australian flying-foxes (Pteropus spp., Megachiroptera)". Australian Zoologist. 32: 76–100. doi:10.7882/AZ.2002.008.
- ^ Martin, L (2011). "Is the fruit you eat flying-fox friendly? The effects of orchard electrocution grids on Australian flying-foxes (Pteropus spp., Megachiroptera)". The Biology and Conservation of Australasian Bats. pp. 380–390. doi:10.7882/FS.2011.039. ISBN 978-0-9803272-4-3.
- ^ Chlopicki, K. (28 October 2016). "Electric wires threaten flying foxes and their new babies". The Daily Telegraph. News Pty Ltd. Retrieved 26 June 2018.
- ^ Welbergen, J. A; Klose, S. M; Markus, N; Eby, P (2008). "Climate change and the effects of temperature extremes on Australian flying-foxes" (PDF). Proceedings of the Royal Society B: Biological Sciences. 275 (1633): 419–25. doi:10.1098/rspb.2007.1385. PMC 2596826. PMID 18048286.
- ^ Buden, Don; Helgen, Kristofer M; Wiles, Gary (2013). "Taxonomy, distribution, and natural history of flying foxes (Chiroptera, Pteropodidae) in the Mortlock Islands and Chuuk State, Caroline Islands". ZooKeys. 345 (345): 97–135. doi:10.3897/zookeys.345.5840. PMC 3817444. PMID 24194666.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fleming, Theodore H.; Racey, Paul A., eds. (2010). Island Bats: Evolution, Ecology, and Conservation. University of Chicago Press. p. 415. ISBN 9780226253312.
- ^ Mickleburgh, Simon; Waylen, Kerry; Racey, Paul (2009). "Bats as bushmeat: A global review". Oryx. 43 (2): 217. doi:10.1017/s0030605308000938.
- ^ Banack, Sandra Anne; Murch, Susan J; Cox, Paul Alan (2006). "Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands". Journal of Ethnopharmacology. 106 (1): 97–104. doi:10.1016/j.jep.2005.12.032. PMID 16457975.
- ^ Cox, P., Davis, D., Mash, D., Metcalf J.S., Banack, S. A. (2016). "Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain". Proceedings of the Royal Society B. 283 (3): 1–10. doi:10.1098/rspb.2015.2397. PMC 4795023. PMID 26791617.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holtcamp, W. (2012). "The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease?". Environmental Health Perspectives. 120 (1823): a110–a116. doi:10.1289/ehp.120-a110. PMC 3295368. PMID 22382274.
- ^ Hassanin, Alexandre; Nesi, Nicolas; Marin, Julie; Kadjo, Blaise; Pourrut, Xavier; Leroy, Éric; Gembu, Guy-Crispin; Musaba Akawa, Prescott; Ngoagouni, Carine; Nakouné, Emmanuel; Ruedi, Manuel; Tshikung, Didier; Pongombo Shongo, Célestin; Bonillo, Céline (2016). "Comparative phylogeography of African fruit bats (Chiroptera, Pteropodidae) provide new insights into the outbreak of Ebola virus disease in West Africa, 2014–2016". Comptes Rendus Biologies. 339 (11–12): 517–528. doi:10.1016/j.crvi.2016.09.005. PMID 27746072.
- ^ "Deadly Marburg virus discovered in fruit bats". msnbc. 21 August 2007. Retrieved 11 March 2008.
- ^ Quammen, David (October 2007). "Deadly Contact". National Geographic: 78–105.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Rabies and Australian bat lyssavirus infection fact sheet". health.nsw.gov.au. State of New South Wales NSW Ministry of Health 2015. 30 November 2015. Retrieved 14 June 2018.
- ^ "Hendra Virus Disease(HeV)" (PDF). cdc.gov. U.S. Department of Health & Human Services. Retrieved 14 June 2018.
- ^ Sánchez, Cecilia A; Baker, Michelle L (2016). "Disease Risk Perception and Safety Practices: A Survey of Australian Flying Fox Rehabilitators". PLOS Neglected Tropical Diseases. 10 (2): e0004411. doi:10.1371/journal.pntd.0004411. PMID 26829399.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "The Hendra vaccine". ava.com. The Australian Veterinary Association Ltd (AVA). 2018. Retrieved 14 June 2018.
- ^ Gulland, Anne (12 June 2018). "Nipah virus 'under control' in India – but Britain and the world must be alert for signs of infected travellers". The Telegraph. Telegraph Media Group Limited 2018. Retrieved 14 June 2018.
- ^ "Nipah virus". World Health Organization. WHO. 30 May 2018. Retrieved 14 June 2018.
- ^ Hahn, M. B.; Epstein, J. H.; Gurley, E. S.; Islam, M. S.; Luby, S. P.; Daszak, P.; Patz, J. A. (2014). "Roosting behaviour and habitat selection of Pteropus giganteus reveal potential links to Nipah virus epidemiology". Journal of Applied Ecology. 51 (2): 376–387. doi:10.1111/1365-2664.12212. PMC 4000083. PMID 24778457.
- ^ a b Bowden, Timothy R; Westenberg, Marcel; Wang, Lin-Fa; Eaton, Bryan T; Boyle, David B (2001). "Molecular Characterization of Menangle Virus, a Novel Paramyxovirus which Infects Pigs, Fruit Bats, and Humans". Virology. 283 (2): 358. doi:10.1006/viro.2001.0893. PMID 11336561.
- ^ a b Yamanaka, Atsushi; Iwakiri, Akira; Yoshikawa, Tomoki; Sakai, Kouji; Singh, Harpal; Himeji, Daisuke; Kikuchi, Ikuo; Ueda, Akira; Yamamoto, Seigo; Miura, Miho; Shioyama, Yoko; Kawano, Kimiko; Nagaishi, Tokiko; Saito, Minako; Minomo, Masumi; Iwamoto, Naoyasu; Hidaka, Yoshio; Sohma, Hirotoshi; Kobayashi, Takeshi; Kanai, Yuta; Kawagishi, Takehiro; Nagata, Noriyo; Fukushi, Shuetsu; Mizutani, Tetsuya; Tani, Hideki; Taniguchi, Satoshi; Fukuma, Aiko; Shimojima, Masayuki; Kurane, Ichiro; et al. (2014). "Imported Case of Acute Respiratory Tract Infection Associated with a Member of Species Nelson Bay Orthoreovirus". PLOS One. 9 (3): e92777. Bibcode:2014PLoSO...992777Y. doi:10.1371/journal.pone.0092777. PMC 3965453. PMID 24667794.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Smith, Ina; Wang, Lin-Fa (2013). "Bats and their virome: An important source of emerging viruses capable of infecting humans". Current Opinion in Virology. 3 (1): 84–91. doi:10.1016/j.coviro.2012.11.006. PMID 23265969.




