Zooplankton: Difference between revisions
Epipelagic (talk | contribs) Microzooplankton – added copy from marine microorganisms – see that page's history for attribution |
Epipelagic (talk | contribs) Dinoflagellates and mixotrophs – added copy from marine microorganisms – see that page's history for attribution |
||
| Line 80: | Line 80: | ||
''Microzooplankton: major grazers of the plankton...'' |
''Microzooplankton: major grazers of the plankton...'' |
||
===Protists=== |
|||
{{further|marine protists}} |
|||
====Radiolarians==== |
====Radiolarians==== |
||
| Line 202: | Line 205: | ||
{{clear}} |
{{clear}} |
||
====Dinoflagellates==== |
|||
{{see also|Predatory dinoflagellate}} |
|||
[[Dinoflagellate]]s are part of the [[#Algae|algae group]], and form a phylum of unicellular flagellates with about 2,000 marine species.<ref name="Gómez12">{{cite journal|author=Gómez F |title=A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata) |journal=CICIMAR Océanides |volume=27 |issue=1 |pages=65–140 |year=2012 |url=http://www.cicimar.ipn.mx/oacis/Medios/oceanides/P%20065%20Fernando%20Gomez.pdf |url-status=dead |archive-url=https://web.archive.org/web/20131127104918/http://www.cicimar.ipn.mx/oacis/Medios/oceanides/P%20065%20Fernando%20Gomez.pdf |archive-date=2013-11-27}}</ref> The name comes from the Greek "dinos" meaning ''whirling'' and the Latin "flagellum" meaning a ''whip'' or ''lash''. This refers to the two whip-like attachments (flagella) used for forward movement. Most dinoflagellates are protected with red-brown, cellulose armour. Like other phytoplankton, dinoflagellates are [[r-strategist]]s which under right conditions can [[Algal bloom|bloom]] and create [[red tide]]s. [[Excavata|Excavates]] may be the most basal flagellate lineage.<ref name=Dawson2013>{{cite journal|last1=Dawson|first1=Scott C|last2=Paredez|first2=Alexander R|title=Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists|journal=Current Opinion in Cell Biology|year=2013|volume=25|issue=1|pages=134–141|doi=10.1016/j.ceb.2012.11.005|pmid=23312067|pmc=4927265}}</ref> |
|||
{{multiple image |
|||
| align = left |
|||
| direction = horizontal |
|||
| width = 120 |
|||
| header = Dinoflagellates |
|||
| header_align = center |
|||
| header_background = |
|||
| footer = Traditionally dinoflagellates have been presented as armoured or unarmoured |
|||
| footer_align = center |
|||
| footer_background = |
|||
| background color = |
|||
| image1 = Peridinium digitale.jpg |
|||
| alt1 = |
|||
| caption1 = Armoured |
|||
| image2 = Gymnodinium agile sp.jpg |
|||
| alt2 = |
|||
| caption2 = Unarmoured |
|||
}} |
|||
<gallery mode=packed heights=144px style=float:right;> |
|||
File:Gyrodinium dinoflagellate.jpg|''[[Gymnodinium|Gyrodinium]]'', one of the few naked dinoflagellates which lack armour |
|||
File:Protoperidinium dinoflagellate.jpg|The dinoflagellate ''Protoperidinium'' extrudes a large feeding veil to capture prey |
|||
File:Radiolarian - Podocyrtis (Lampterium) mitra Ehrenberg - 160x.jpg|[[Nassellarian]] radiolarians can be in symbiosis with dinoflagellates |
|||
</gallery> |
|||
{{clear}} |
|||
Dinoflagellates often live in [[symbiosis]] with other organisms. Many [[nassellarian]] radiolarians house [[dinoflagellate]] [[Symbiosis|symbionts]] within their tests.<ref>{{Cite book|title=Handbook of the Protists|last1=Boltovskoy|first1=Demetrio|last2=Anderson|first2=O. Roger|last3=Correa|first3=Nancy M.|date=2017|publisher=Springer, Cham|isbn=9783319281476|pages=731–763|language=en|doi=10.1007/978-3-319-28149-0_19}}</ref> The nassellarian provides [[ammonium]] and [[carbon dioxide]] for the dinoflagellate, while the dinoflagellate provides the nassellarian with a mucous membrane useful for hunting and protection against harmful invaders.<ref>{{Cite book|title=Radiolaria|last=Anderson|first=O. R.|publisher=Springer Science & Business Media|year=1983|isbn=|location=|pages=}}</ref> There is evidence from [[DNA]] analysis that dinoflagellate symbiosis with radiolarians evolved independently from other dinoflagellate symbioses, such as with [[foraminifera]].<ref>{{Cite journal|last1=Gast|first1=R. J.|last2=Caron|first2=D. A.|date=1996-11-01|title=Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria|journal=Molecular Biology and Evolution|language=en|volume=13|issue=9|pages=1192–1197|doi=10.1093/oxfordjournals.molbev.a025684|pmid=8896371|issn=0737-4038|doi-access=free}}</ref> |
|||
<gallery mode=packed heights=144px style=float:left;> |
|||
File:Ceratium tripos.jpg| [[Tripos (dinoflagellate)|''Tripos muelleri'']] is recognisable by its U-shaped horns |
|||
File:Archives de zoologie expérimentale et générale (1920) (20299351186).jpg|''[[Oodinium]]'', a genus of [[parasitic]] dinoflagellates, causes [[velvet disease]] in fish<ref>{{cite web|title=Protozoa Infecting Gills and Skin|url=http://www.merckvetmanual.com:80/mvm/index.jsp?cfile=htm/bc/170410.htm|publisher=[[The Merck Veterinary Manual]]|accessdate= 4 November 2019|archiveurl=https://web.archive.org/web/20160303221140/http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm%2Fbc%2F170410.htm|archivedate=3 March 2016|url-status=dead|df=dmy-all}}</ref> |
|||
File:Karenia brevis.jpg|''[[Karenia brevis]]'' produces red tides highly toxic to humans<ref>{{Cite journal|last1=Brand|first1=Larry E.|last2=Campbell|first2=Lisa|last3=Bresnan|first3=Eileen|title=''Karenia'': The biology and ecology of a toxic genus|journal=Harmful Algae|volume=14|pages=156–178|doi=10.1016/j.hal.2011.10.020|year=2012}}</ref> |
|||
File:Algal bloom(akasio) by Noctiluca in Nagasaki.jpg|[[Red tide]] |
|||
</gallery> |
|||
{{clear}} |
|||
====Mixotrophs==== |
|||
{{see also|Mixotroph|Mixotrophic dinoflagellate}} |
|||
{{multiple image |
|||
| align = right |
|||
| direction = horizontal |
|||
| header = Mixotrophic radiolarians |
|||
| header_align = center |
|||
| header_background = |
|||
| footer = |
|||
| footer_align = center |
|||
| footer_background = |
|||
| background color = |
|||
| width1 = 170 |
|||
| image1 = Phaeocystis symbionts within an acantharian host.png |
|||
| alt1 = |
|||
| caption1 = [[Acantharian]] radiolarian hosts ''[[Phaeocystis]]'' symbionts |
|||
| width2 = 200 |
|||
| image2 = Ecomare - schuimalg strand (7037-schuimalg-phaeocystis-ogb).jpg |
|||
| alt2 = |
|||
| caption2 = White ''Phaeocystis'' algal foam washing up on a beach |
|||
}} |
|||
A [[mixotroph]] is an organism that can use a mix of different [[Primary nutritional groups|sources of energy and carbon]], instead of having a single trophic mode on the continuum from complete [[autotrophy]] at one end to [[heterotrophy]] at the other. It is estimated that mixotrophs comprise more than half of all microscopic plankton.<ref>[https://www.irishexaminer.com/lifestyle/outdoors/richard-collins/beware-the-mixotrophs--they-can-destroy-entire-ecosystems-in-a-matter-of-hours-430358.html Beware the mixotrophs - they can destroy entire ecosystems 'in a matter of hours']</ref> There are two types of eukaryotic mixotrophs: those with their own [[chloroplast]]s, and those with [[endosymbiont]]s—and others that acquire them through [[kleptoplasty]] or by enslaving the entire phototrophic cell.<ref>[https://phys.org/news/2017-08-microscopic-body-snatchers-infest-oceans.html Microscopic body snatchers infest our oceans - Phys.org]</ref> |
|||
The distinction between plants and animals often breaks down in very small organisms. Possible combinations are [[phototroph|photo-]] and [[chemotroph]]y, [[lithotroph|litho-]] and [[organotroph]]y, [[autotroph|auto-]] and [[heterotroph]]y or other combinations of these. Mixotrophs can be either [[eukaryote|eukaryotic]] or [[prokaryote|prokaryotic]].<ref name='Eiler'>{{cite journal |author= Eiler A |title= Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences |journal=Appl Environ Microbiol |volume=72 |issue=12 |pages= 7431–7|date=December 2006|pmid=17028233 |doi=10.1128/AEM.01559-06|pmc=1694265}}</ref> They can take advantage of different environmental conditions.<ref>{{cite journal |vauthors=Katechakis A, Stibor H |title=The mixotroph ''Ochromonas tuberculata'' may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions |journal= Oecologia|volume= 148 |issue= 4|pages=692–701 |date=July 2006|pmid=16568278 |doi= 10.1007/s00442-006-0413-4|bibcode=2006Oecol.148..692K }}</ref> |
|||
Recent studies of marine microzooplankton found 30–45% of the ciliate abundance was mixotrophic, and up to 65% of the amoeboid, foram and radiolarian [[Biomass (ecology)|biomass]] was mixotrophic.<ref name=Leles2017>{{cite journal | last1 = Leles | first1 = S.G. | last2 = Mitra | first2 = A. | last3 = Flynn | first3 = K.J. | last4 = Stoecker | first4 = D.K. | last5 = Hansen | first5 = P.J. | last6 = Calbet | first6 = A. | last7 = McManus | first7 = G.B. | last8 = Sanders | first8 = R.W. | last9 = Caron | first9 = D.A. | last10 = Not | first10 = F. | last11 = Hallegraeff | first11 = G.M. | year = 2017 | title = Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance | url = | journal = Proceedings of the Royal Society B: Biological Sciences | volume = 284 | issue = 1860| page = 20170664 | doi = 10.1098/rspb.2017.0664 | pmid = 28768886 | pmc = 5563798 }}</ref> |
|||
''[[Phaeocystis]]'' is an important algal genus found as part of the marine [[phytoplankton]] around the world. It has a [[Polymorphism (biology)|polymorphic]] life cycle, ranging from free-living cells to large colonies.<ref name=":0">{{Cite journal|title = Phaeocystis blooms in the global ocean and their controlling mechanisms: a review|journal = Journal of Sea Research|date = 2005-01-01|pages = 43–66|volume = 53|series = Iron Resources and Oceanic Nutrients - Advancement of Global Environmental Simulations|issue = 1–2|doi = 10.1016/j.seares.2004.01.008|first1 = Véronique|last1 = Schoemann|first2 = Sylvie|last2 = Becquevort|first3 = Jacqueline|last3 = Stefels|first4 = Véronique|last4 = Rousseau|first5 = Christiane|last5 = Lancelot|citeseerx = 10.1.1.319.9563|bibcode = 2005JSR....53...43S}}</ref> It has the ability to form floating colonies, where hundreds of cells are embedded in a gel matrix, which can increase massively in size during [[Algal bloom|blooms]].<ref>{{cite web |url= http://www.phaeocystis.org/|title = Welcome to the Phaeocystis antarctica genome sequencing project homepage |date= |accessdate= |website= |last= |first=}}</ref> As a result, ''Phaeocystis'' is an important contributor to the marine [[Carbon cycle|carbon]]<ref>{{cite journal |title=Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica <!--http://www.nature.com/doifinder/10.1038/35007061-->|journal = Nature|pages = 595–598|volume = 404|issue = 6778|doi = 10.1038/35007061|pmid = 10766240|first1 = G. R.|last1 = DiTullio|first2 = J. M.|last2 = Grebmeier|first3 = K. R.|last3 = Arrigo|first4 = M. P.|last4 = Lizotte|first5 = D. H.|last5 = Robinson|first6 = A.|last6 = Leventer|first7 = J. P.|last7 = Barry|first8 = M. L.|last8 = VanWoert|first9 = R. B.|last9 = Dunbar|year = 2000}}</ref> and [[sulfur cycle]]s.<ref>{{Cite journal|title = DMSP-lyase activity in a spring phytoplankton bloom off the Dutch coast, related to Phaeocystis sp. abundance|journal = Marine Ecology Progress Series|date = 1995-07-20|pages = 235–243|volume = 123|doi = 10.3354/meps123235|first1 = Stefels|last1 = J|first2 = Dijkhuizen|last2 = L|first3 = Gieskes|last3 = WWC|url = https://pure.rug.nl/ws/files/62552225/DMSP_lyase_activity_in_a_spring_phytoplankton_bloom_off_the_Dutch_coast.pdf|bibcode = 1995MEPS..123..235S}}</ref> ''Phaeocystis'' species are endosymbionts to [[Acantharea|acantharian]] radiolarians.<ref name=":4">{{cite journal|last1=Decelle|first1=Johan|last2=Simó|first2=Rafel|last3=Galí|first3=Martí|last4=Vargas|first4=Colomban de|last5=Colin|first5=Sébastien|last6=Desdevises|first6=Yves|last7=Bittner|first7=Lucie|last8=Probert|first8=Ian|last9=Not|first9=Fabrice|date=2012-10-30|title=An original mode of symbiosis in open ocean plankton|journal=Proceedings of the National Academy of Sciences|language=en|volume=109|issue=44|pages=18000–18005|doi=10.1073/pnas.1212303109|issn=0027-8424|pmid=23071304|pmc=3497740|bibcode=2012PNAS..10918000D}}</ref><ref name=":5">{{Cite journal|last1=Mars Brisbin|first1=Margaret|last2=Grossmann|first2=Mary M.|last3=Mesrop|first3=Lisa Y.|last4=Mitarai|first4=Satoshi|date=2018|title=Intra-host Symbiont Diversity and Extended Symbiont Maintenance in Photosymbiotic Acantharea (Clade F)|journal=Frontiers in Microbiology|language=English|volume=9|pages=1998|doi=10.3389/fmicb.2018.01998|pmid=30210473|pmc=6120437|issn=1664-302X}}</ref> |
|||
{|class="wikitable" |
|||
! colspan=7 |{{centre|Mixotrophic zooplankton that combine phototrophy and heterotrophy – table based on Stoecker et. al., 2017 <ref name=Stoecker2017>{{cite journal | last1 = Stoecker | first1 = D.K. | last2 = Hansen | first2 = P.J. | last3 = Caron | first3 = D.A. | last4 = Mitra | first4 = A. | year = 2017 | title = Mixotrophy in the marine plankton | url = | journal = Annual Review of Marine Science | volume = 9 | issue = | pages = 311–335 | doi = 10.1146/annurev-marine-010816-060617 | pmid = 27483121 | bibcode = 2017ARMS....9..311S }}</ref>}} |
|||
|- |
|||
! colspan=2 | Description |
|||
! colspan=2 | Example |
|||
! Further examples |
|||
|- |
|||
| colspan=5 | Called '''''nonconstitutive mixotrophs''''' by Mitra et. al., 2016.<ref name=Mitra2016>{{cite journal | last1 = Mitra | first1 = A | last2 = Flynn | first2 = KJ | last3 = Tillmann | first3 = U | last4 = Raven | first4 = J | last5 = Caron | first5 = D | display-authors = etal | year = 2016 | title = Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition; incorporation of diverse mixotrophic strategies | url = | journal = Protist | volume = 167 | issue = 2| pages = 106–20 | doi = 10.1016/j.protis.2016.01.003 | pmid = 26927496 | doi-access = free }}</ref> Zooplankton that are photosynthetic: microzooplankton or metazoan zooplankton that acquire phototrophy through chloroplast retention<sup>a</sup> or maintenance of algal endosymbionts. |
|||
|- |
|||
| Generalists |
|||
| Protists that retain chloroplasts and rarely other organelles from many algal taxa |
|||
| [[File:Halteria.jpg|100px]] |
|||
| |
|||
| Most [[oligotrich]] ciliates that retain plastids<sup>a</sup> |
|||
|- |
|||
| rowspan=2 | Specialists |
|||
| 1. Protists that retain chloroplasts and sometimes other organelles from one algal species or very closely related algal species |
|||
| [[File:Dinophysis acuminata.jpg|100px]] |
|||
| ''[[Dinophysis acuminata]]'' |
|||
| ''[[Dinophysis]]'' spp.<br />''[[Myrionecta rubra]]'' |
|||
|- |
|||
| 2. Protists or zooplankton with algal endosymbionts of only one algal species or very closely related algal species |
|||
| [[File:Noctiluca scintillans varias.jpg|100px]] |
|||
| ''[[Noctiluca scintillans]]'' |
|||
| [[wiktionary:metazooplankton|Metazooplankton]] with algal [[endosymbiont]]s<br />Most mixotrophic [[Rhizaria]] ([[Acantharea]], [[Polycystinea]], and [[Foraminifera]])<br />Green ''[[Noctiluca scintillans]]'' |
|||
|- |
|||
| colspan=7 | <center><small><sup>a</sup>Chloroplast (or plastid) retention = sequestration = enslavement. Some plastid-retaining species also retain other organelles and prey cytoplasm.</small></center> |
|||
|} |
|||
<gallery caption="Mixoplankton" mode=packed heights=144px style=float:left;> |
|||
File:Tintinnid ciliate Favella.jpg|[[Tintinnid]] ciliate ''Favella'' |
|||
File:Euglena mutabilis - 400x - 1 (10388739803) (cropped).jpg|''[[Euglena|Euglena mutabilis]]'', a photosynthetic [[flagellate]] |
|||
File:Stichotricha secunda - 400x (14974779356).jpg|[[Zoochlorellae]] (green) living inside the [[ciliate]] ''Stichotricha secunda'' |
|||
File:Dinophysis acuta.tif| The dinoflagellate ''Dinophysis acuta'' |
|||
</gallery> |
|||
{{clear}} |
|||
By trophic orientation dinoflagellates are all over the place. Some dinoflagellates are known to be [[photosynthesis|photosynthetic]], but a large fraction of these are in fact [[mixotrophy|mixotrophic]], combining photosynthesis with ingestion of prey ([[phagotrophy]]).<ref>{{Cite journal | last1 = Stoecker | first1 = D. K. | title = Mixotrophy among Dinoflagellates | doi = 10.1111/j.1550-7408.1999.tb04619.x | journal = The Journal of Eukaryotic Microbiology | volume = 46 | issue = 4 | pages = 397–401 | year = 1999 | pmid = | pmc = | name-list-format = vanc}}</ref> Some species are [[endosymbiont]]s of marine animals and other protists, and play an important part in the biology of [[coral reef]]s. Others predate other protozoa, and a few forms are parasitic. Many dinoflagellates are [[mixotrophic]] and could also be classified as phytoplankton. |
|||
The toxic dinoflagellate ''[[Dinophysis acuta]]'' acquire chloroplasts from its prey. "It cannot catch the cryptophytes by |
|||
itself, and instead relies on ingesting ciliates such as the red ''[[Myrionecta rubra]]'', which sequester their chloroplasts from a |
|||
specific cryptophyte clade (Geminigera/Plagioselmis/Teleaulax)".<ref name=Stoecker2017 /> |
|||
==Zooplankton diversity== |
==Zooplankton diversity== |
||
Revision as of 05:45, 23 August 2020

Zooplankton (/ˈzoʊ.əˌplæŋktən, ˈzuː(ə)-, ˈzoʊoʊ-/,[1] /ˌzoʊ.əˈplæŋktən, -tɒn/)[2] are heterotrophic (sometimes detritivorous) plankton (cf. phytoplankton). Plankton are organisms drifting in oceans, seas, and bodies of fresh water. The word zooplankton is derived from the Greek zoon (ζῴον), meaning "animal", and planktos (πλαγκτός), meaning "wanderer" or "drifter".[3] Individual zooplankton are usually microscopic, but some (such as jellyfish) are larger and visible to the naked eye.
Ecology
Zooplankton is a categorization spanning a range of organism sizes including small protozoans and large metazoans. It includes holoplanktonic organisms whose complete life cycle lies within the plankton, as well as meroplanktonic organisms that spend part of their lives in the plankton before graduating to either the nekton or a sessile, benthic existence. Although zooplankton are primarily transported by ambient water currents, many have locomotion, used to avoid predators (as in diel vertical migration) or to increase prey encounter rate.
- Typical models featuring zooplankton
-
Upper left: Biogeochemical modelsRight: Ecosystem modelsLower left: Size-spectra modelsThese models also have temporal and spatial components.[4]
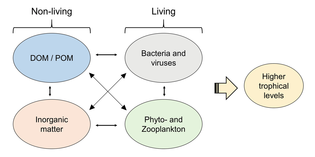
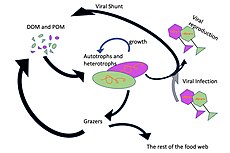
Ecologically important protozoan zooplankton groups include the foraminiferans, radiolarians and dinoflagellates (the last of these are often mixotrophic). Important metazoan zooplankton include cnidarians such as jellyfish and the Portuguese Man o' War; crustaceans such as copepods, ostracods, isopods, amphipods, mysids and krill; chaetognaths (arrow worms); molluscs such as pteropods; and chordates such as salps and juvenile fish. This wide phylogenetic range includes a similarly wide range in feeding behavior: filter feeding, predation and symbiosis with autotrophic phytoplankton as seen in corals. Zooplankton feed on bacterioplankton, phytoplankton, other zooplankton (sometimes cannibalistically), detritus (or marine snow) and even nektonic organisms. As a result, zooplankton are primarily found in surface waters where food resources (phytoplankton or other zooplankton) are abundant.
Just as any species can be limited within a geographical region, so are zooplankton. However, species of zooplankton are not dispersed uniformly or randomly within a region of the ocean. As with phytoplankton, ‘patches’ of zooplankton species exist throughout the ocean. Though few physical barriers exist above the mesopelagic, specific species of zooplankton are strictly restricted by salinity and temperature gradients; while other species can withstand wide temperature and salinity gradients.[5] Zooplankton patchiness can also be influenced by biological factors, as well as other physical factors. Biological factors include breeding, predation, concentration of phytoplankton, and vertical migration.[5] The physical factor that influences zooplankton distribution the most is mixing of the water column (upwelling and downwelling along the coast and in the open ocean) that affects nutrient availability and, in turn, phytoplankton production.[5]
Through their consumption and processing of phytoplankton and other food sources, zooplankton play a role in aquatic food webs, as a resource for consumers on higher trophic levels (including fish), and as a conduit for packaging the organic material in the biological pump. Since they are typically small, zooplankton can respond rapidly to increases in phytoplankton abundance,[clarification needed] for instance, during the spring bloom. Zooplankton are also a key link in the biomagnification of pollutants such as mercury.[6]
Zooplankton can also act as a disease reservoir. Crustacean zooplankton have been found to house the bacterium Vibrio cholerae, which causes cholera, by allowing the cholera vibrios to attach to their chitinous exoskeletons. This symbiotic relationship enhances the bacterium's ability to survive in an aquatic environment, as the exoskeleton provides the bacterium with carbon and nitrogen.[7]
Role in food webs

- Pelagic food web
-
Pelagic food web and the biological pump. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the euphotic zone, while the darker blue waters represent the twilight zone.[11]
Role in biogeochemistry
Microzooplankton
Microzooplankton: major grazers of the plankton...
Protists
Radiolarians
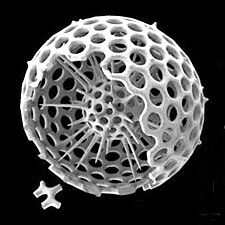
Radiolarians are unicellular predatory protists encased in elaborate globular shells usually made of silica and pierced with holes. Their name comes from the Latin for "radius". They catch prey by extending parts of their body through the holes. As with the silica frustules of diatoms, radiolarian shells can sink to the ocean floor when radiolarians die and become preserved as part of the ocean sediment. These remains, as microfossils, provide valuable information about past oceanic conditions.[13]
-
Like diatoms, radiolarians come in many shapes
-
Also like diatoms, radiolarian shells are usually made of silicate
-
However acantharian radiolarians have shells made from strontium sulfate crystals
-
Cutaway schematic diagram of a spherical radiolarian shell
| External videos | |
|---|---|
Foraminiferans
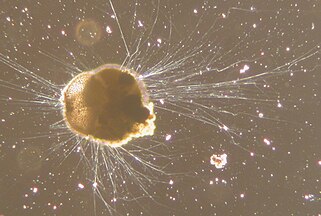
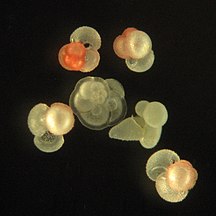
Like radiolarians, foraminiferans (forams for short) are single-celled predatory protists, also protected with shells that have holes in them. Their name comes from the Latin for "hole bearers". Their shells, often called tests, are chambered (forams add more chambers as they grow). The shells are usually made of calcite, but are sometimes made of agglutinated sediment particles or chiton, and (rarely) of silica. Most forams are benthic, but about 40 species are planktic.[14] They are widely researched with well established fossil records which allow scientists to infer a lot about past environments and climates.[13]
-
section showing chambers of a spiral foram
-
Live Ammonia tepida streaming granular ectoplasm for catching food
-
Group of planktonic forams
| External videos | |
|---|---|
A number of forams are mixotrophic (see below). These have unicellular algae as endosymbionts, from diverse lineages such as the green algae, red algae, golden algae, diatoms, and dinoflagellates.[14] Mixotrophic foraminifers are particularly common in nutrient-poor oceanic waters.[16] Some forams are kleptoplastic, retaining chloroplasts from ingested algae to conduct photosynthesis.[17]
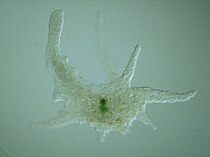
Amoeba
-
Naked amoeba showing food vacuoles and ingested diatom
-
Shell or test of a testate amoeba, Arcella sp.
-
Xenogenic testate amoeba covered in diatoms
Ciliates
-
Holophyra ovum
-
This ciliate is digesting cyanobacteria. The mouth is at the bottom right.
Dinoflagellates
Dinoflagellates are part of the algae group, and form a phylum of unicellular flagellates with about 2,000 marine species.[18] The name comes from the Greek "dinos" meaning whirling and the Latin "flagellum" meaning a whip or lash. This refers to the two whip-like attachments (flagella) used for forward movement. Most dinoflagellates are protected with red-brown, cellulose armour. Like other phytoplankton, dinoflagellates are r-strategists which under right conditions can bloom and create red tides. Excavates may be the most basal flagellate lineage.[19]
-
Gyrodinium, one of the few naked dinoflagellates which lack armour
-
The dinoflagellate Protoperidinium extrudes a large feeding veil to capture prey
-
Nassellarian radiolarians can be in symbiosis with dinoflagellates
Dinoflagellates often live in symbiosis with other organisms. Many nassellarian radiolarians house dinoflagellate symbionts within their tests.[20] The nassellarian provides ammonium and carbon dioxide for the dinoflagellate, while the dinoflagellate provides the nassellarian with a mucous membrane useful for hunting and protection against harmful invaders.[21] There is evidence from DNA analysis that dinoflagellate symbiosis with radiolarians evolved independently from other dinoflagellate symbioses, such as with foraminifera.[22]
-
Tripos muelleri is recognisable by its U-shaped horns
-
Karenia brevis produces red tides highly toxic to humans[24]
Mixotrophs
A mixotroph is an organism that can use a mix of different sources of energy and carbon, instead of having a single trophic mode on the continuum from complete autotrophy at one end to heterotrophy at the other. It is estimated that mixotrophs comprise more than half of all microscopic plankton.[25] There are two types of eukaryotic mixotrophs: those with their own chloroplasts, and those with endosymbionts—and others that acquire them through kleptoplasty or by enslaving the entire phototrophic cell.[26]
The distinction between plants and animals often breaks down in very small organisms. Possible combinations are photo- and chemotrophy, litho- and organotrophy, auto- and heterotrophy or other combinations of these. Mixotrophs can be either eukaryotic or prokaryotic.[27] They can take advantage of different environmental conditions.[28]
Recent studies of marine microzooplankton found 30–45% of the ciliate abundance was mixotrophic, and up to 65% of the amoeboid, foram and radiolarian biomass was mixotrophic.[29]
Phaeocystis is an important algal genus found as part of the marine phytoplankton around the world. It has a polymorphic life cycle, ranging from free-living cells to large colonies.[30] It has the ability to form floating colonies, where hundreds of cells are embedded in a gel matrix, which can increase massively in size during blooms.[31] As a result, Phaeocystis is an important contributor to the marine carbon[32] and sulfur cycles.[33] Phaeocystis species are endosymbionts to acantharian radiolarians.[34][35]
Mixotrophic zooplankton that combine phototrophy and heterotrophy – table based on Stoecker et. al., 2017 [36]
| ||||||
|---|---|---|---|---|---|---|
| Description | Example | Further examples | ||||
| Called nonconstitutive mixotrophs by Mitra et. al., 2016.[37] Zooplankton that are photosynthetic: microzooplankton or metazoan zooplankton that acquire phototrophy through chloroplast retentiona or maintenance of algal endosymbionts. | ||||||
| Generalists | Protists that retain chloroplasts and rarely other organelles from many algal taxa | 
|
Most oligotrich ciliates that retain plastidsa | |||
| Specialists | 1. Protists that retain chloroplasts and sometimes other organelles from one algal species or very closely related algal species | 
|
Dinophysis acuminata | Dinophysis spp. Myrionecta rubra | ||
| 2. Protists or zooplankton with algal endosymbionts of only one algal species or very closely related algal species | 
|
Noctiluca scintillans | Metazooplankton with algal endosymbionts Most mixotrophic Rhizaria (Acantharea, Polycystinea, and Foraminifera) Green Noctiluca scintillans | |||
- Mixoplankton
-
Tintinnid ciliate Favella
-
Euglena mutabilis, a photosynthetic flagellate
-
Zoochlorellae (green) living inside the ciliate Stichotricha secunda
-
The dinoflagellate Dinophysis acuta
By trophic orientation dinoflagellates are all over the place. Some dinoflagellates are known to be photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey (phagotrophy).[38] Some species are endosymbionts of marine animals and other protists, and play an important part in the biology of coral reefs. Others predate other protozoa, and a few forms are parasitic. Many dinoflagellates are mixotrophic and could also be classified as phytoplankton.
The toxic dinoflagellate Dinophysis acuta acquire chloroplasts from its prey. "It cannot catch the cryptophytes by itself, and instead relies on ingesting ciliates such as the red Myrionecta rubra, which sequester their chloroplasts from a specific cryptophyte clade (Geminigera/Plagioselmis/Teleaulax)".[36]
Zooplankton diversity
- Ichthyoplankton
-
Salmon eggs
-
Juvenile planktonic squid
-
Ocean sunfish larvae (2.7mm)
-
Boxfish larva
See also
- Bacterioplankton
- Biological pump
- Census of Marine Zooplankton
- Diel vertical migration
- Gelatinous zooplankton
- Ocean acidification
- Phytoplankton
- Plankton
- Primary production
- Thin layers (oceanography)
References
- ^ "zooplankton - definition of zooplankton in English from the Oxford dictionary". OxfordDictionaries.com. Retrieved 2016-01-20.
- ^ "Zooplankton". Merriam-Webster.com Dictionary.
- ^ Thurman, H. V. (1997). Introductory Oceanography. New Jersey, USA: Prentice Hall College. ISBN 978-0-13-262072-7.
- ^ Everett, J.D., Baird, M.E., Buchanan, P., Bulman, C., Davies, C., Downie, R., Griffiths, C., Heneghan, R., Kloser, R.J., Laiolo, L. and Lara-Lopez, A. (2017) "Modeling what we sample and sampling what we model: challenges for zooplankton model assessment". Frontiers in Marine Science, 4: 77. doi:10.3389/fmars.2017.00077.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c Lalli, C.M.; Parsons, T.R. (1993). Biological Oceanography An Introduction. Burlington, MA: Elsevier. p. 314. ISBN 978-0-7506-3384-0.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ "How We Do Things at IISD-ELA: Researching Mercury". IISD. 2017-04-05. Retrieved 2020-07-06.
- ^ Jude, B.A.; Kirn, T.J.; Taylor R.K. (2005). "A colonization factor links Vibrio cholerae environmental survival and human infection". Nature. 438 (7069): 863–6. Bibcode:2005Natur.438..863K. doi:10.1038/nature04249. PMID 16341015.
- ^ Møller EF, Thor P, Nielson TG (2003) "Production of DOC by Calanus finmarchicus, C. glacialis and C. hyperboreus through sloppy feeding and leakage from fecal pellets". Marine Ecology Progress Series, 262: 185–91. doi:10.3354/meps262185.
- ^ Saba GK, Steinberg DK, Bronk DA (2009) "Effects of diet on release of dissolved organic and inorganic nutrients by the copepod Acartia tonsa". Marine Ecology Progress Series, 386: 147–61. doi:10.3354/meps08070.
- ^ Steinberg, D.K. and Landry, M.R. (2017) "Zooplankton and the ocean carbon cycle". Annual Review of Marine Science, 9: 413–444. doi:10.1146/annurev-marine-010814-015924.
- ^ Siegel, David A.; Buesseler, Ken O.; Behrenfeld, Michael J.; Benitez-Nelson, Claudia R.; Boss, Emmanuel; Brzezinski, Mark A.; Burd, Adrian; Carlson, Craig A.; d'Asaro, Eric A.; Doney, Scott C.; Perry, Mary J.; Stanley, Rachel H. R.; Steinberg, Deborah K. (2016). "Prediction of the Export and Fate of Global Ocean Net Primary Production: The EXPORTS Science Plan". Frontiers in Marine Science. 3. doi:10.3389/fmars.2016.00022.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Heinrichs, M.E., Mori, C. and Dlugosch, L. (2020) "Complex Interactions Between Aquatic Organisms and Their Chemical Environment Elucidated from Different Perspectives". In: YOUMARES 9-The Oceans: Our Research, Our Future , pages 279–297. Springer. doi:10.1007/978-3-030-20389-4_15.
- ^ a b Wassilieff, Maggy (2006) "Plankton - Animal plankton", Te Ara - the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ^ a b Hemleben, C.; Anderson, O.R.; Spindler, M. (1989). Modern Planktonic Foraminifera. Springer-Verlag. ISBN 978-3-540-96815-3.
- ^ Foraminifera: History of Study, University College London. Retrieved: 18 November 2019.
- ^ Advances in Microbial Ecology, Volum 11
- ^ Bernhard, J. M.; Bowser, S.M. (1999). "Benthic Foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology". Earth-Science Reviews. 46 (1): 149–165. Bibcode:1999ESRv...46..149B. doi:10.1016/S0012-8252(99)00017-3.
- ^ Gómez F (2012). "A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata)" (PDF). CICIMAR Océanides. 27 (1): 65–140. Archived from the original (PDF) on 2013-11-27.
- ^ Dawson, Scott C; Paredez, Alexander R (2013). "Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists". Current Opinion in Cell Biology. 25 (1): 134–141. doi:10.1016/j.ceb.2012.11.005. PMC 4927265. PMID 23312067.
- ^ Boltovskoy, Demetrio; Anderson, O. Roger; Correa, Nancy M. (2017). Handbook of the Protists. Springer, Cham. pp. 731–763. doi:10.1007/978-3-319-28149-0_19. ISBN 9783319281476.
- ^ Anderson, O. R. (1983). Radiolaria. Springer Science & Business Media.
- ^ Gast, R. J.; Caron, D. A. (1996-11-01). "Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria". Molecular Biology and Evolution. 13 (9): 1192–1197. doi:10.1093/oxfordjournals.molbev.a025684. ISSN 0737-4038. PMID 8896371.
- ^ "Protozoa Infecting Gills and Skin". The Merck Veterinary Manual. Archived from the original on 3 March 2016. Retrieved 4 November 2019.
- ^ Brand, Larry E.; Campbell, Lisa; Bresnan, Eileen (2012). "Karenia: The biology and ecology of a toxic genus". Harmful Algae. 14: 156–178. doi:10.1016/j.hal.2011.10.020.
- ^ Beware the mixotrophs - they can destroy entire ecosystems 'in a matter of hours'
- ^ Microscopic body snatchers infest our oceans - Phys.org
- ^ Eiler A (December 2006). "Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences". Appl Environ Microbiol. 72 (12): 7431–7. doi:10.1128/AEM.01559-06. PMC 1694265. PMID 17028233.
- ^ Katechakis A, Stibor H (July 2006). "The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions". Oecologia. 148 (4): 692–701. Bibcode:2006Oecol.148..692K. doi:10.1007/s00442-006-0413-4. PMID 16568278.
- ^ Leles, S.G.; Mitra, A.; Flynn, K.J.; Stoecker, D.K.; Hansen, P.J.; Calbet, A.; McManus, G.B.; Sanders, R.W.; Caron, D.A.; Not, F.; Hallegraeff, G.M. (2017). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences. 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMC 5563798. PMID 28768886.
- ^ Schoemann, Véronique; Becquevort, Sylvie; Stefels, Jacqueline; Rousseau, Véronique; Lancelot, Christiane (2005-01-01). "Phaeocystis blooms in the global ocean and their controlling mechanisms: a review". Journal of Sea Research. Iron Resources and Oceanic Nutrients - Advancement of Global Environmental Simulations. 53 (1–2): 43–66. Bibcode:2005JSR....53...43S. CiteSeerX 10.1.1.319.9563. doi:10.1016/j.seares.2004.01.008.
- ^ "Welcome to the Phaeocystis antarctica genome sequencing project homepage".
- ^ DiTullio, G. R.; Grebmeier, J. M.; Arrigo, K. R.; Lizotte, M. P.; Robinson, D. H.; Leventer, A.; Barry, J. P.; VanWoert, M. L.; Dunbar, R. B. (2000). "Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica". Nature. 404 (6778): 595–598. doi:10.1038/35007061. PMID 10766240.
- ^ J, Stefels; L, Dijkhuizen; WWC, Gieskes (1995-07-20). "DMSP-lyase activity in a spring phytoplankton bloom off the Dutch coast, related to Phaeocystis sp. abundance" (PDF). Marine Ecology Progress Series. 123: 235–243. Bibcode:1995MEPS..123..235S. doi:10.3354/meps123235.
- ^ Decelle, Johan; Simó, Rafel; Galí, Martí; Vargas, Colomban de; Colin, Sébastien; Desdevises, Yves; Bittner, Lucie; Probert, Ian; Not, Fabrice (2012-10-30). "An original mode of symbiosis in open ocean plankton". Proceedings of the National Academy of Sciences. 109 (44): 18000–18005. Bibcode:2012PNAS..10918000D. doi:10.1073/pnas.1212303109. ISSN 0027-8424. PMC 3497740. PMID 23071304.
- ^ Mars Brisbin, Margaret; Grossmann, Mary M.; Mesrop, Lisa Y.; Mitarai, Satoshi (2018). "Intra-host Symbiont Diversity and Extended Symbiont Maintenance in Photosymbiotic Acantharea (Clade F)". Frontiers in Microbiology. 9: 1998. doi:10.3389/fmicb.2018.01998. ISSN 1664-302X. PMC 6120437. PMID 30210473.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. (2017). "Mixotrophy in the marine plankton". Annual Review of Marine Science. 9: 311–335. Bibcode:2017ARMS....9..311S. doi:10.1146/annurev-marine-010816-060617. PMID 27483121.
- ^ Mitra, A; Flynn, KJ; Tillmann, U; Raven, J; Caron, D; et al. (2016). "Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition; incorporation of diverse mixotrophic strategies". Protist. 167 (2): 106–20. doi:10.1016/j.protis.2016.01.003. PMID 26927496.
- ^ Stoecker, D. K. (1999). "Mixotrophy among Dinoflagellates". The Journal of Eukaryotic Microbiology. 46 (4): 397–401. doi:10.1111/j.1550-7408.1999.tb04619.x.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
External links
- SAHFOS Sir Alister Hardy Foundation for Ocean Science
- Ocean Drifters Short film narrated by David Attenborough about the varied roles of plankton
- Sea Drifters BBC Audio slideshow
- Plankton Chronicles Short documentary films & photos
- COPEPOD: The global plankton database. A global coverage database of zooplankton biomass and abundance data.
- Guide to the marine zooplankton of south eastern Australia, Tasmanian Aquaculture and Fisheries Institute
- Australian Continuous Plankton Recorder Project
- An Image-Based Key to Zooplankton of North America

![Upper left: Biogeochemical models Right: Ecosystem models Lower left: Size-spectra models These models also have temporal and spatial components.[4]](http://upload.wikimedia.org/wikipedia/commons/e/ec/Typical_ocean_models_featuring_zooplankton_2.jpg)
![Pelagic food web and the biological pump. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the euphotic zone, while the darker blue waters represent the twilight zone.[11]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/13/Export_Processes_in_the_Ocean_from_Remote_Sensing.jpg/627px-Export_Processes_in_the_Ocean_from_Remote_Sensing.jpg)






![The Egyptian pyramids were constructed from limestone that contained nummulites.[15]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/af/All_Gizah_Pyramids.jpg/325px-All_Gizah_Pyramids.jpg)














![Oodinium, a genus of parasitic dinoflagellates, causes velvet disease in fish[23]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/Archives_de_zoologie_exp%C3%A9rimentale_et_g%C3%A9n%C3%A9rale_%281920%29_%2820299351186%29.jpg/260px-Archives_de_zoologie_exp%C3%A9rimentale_et_g%C3%A9n%C3%A9rale_%281920%29_%2820299351186%29.jpg)
![Karenia brevis produces red tides highly toxic to humans[24]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a0/Karenia_brevis.jpg/214px-Karenia_brevis.jpg)




















