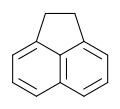
Acenaphthene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2-Dihydroacenaphthylene
| |||
| Other names
1,8-Ethylenenaphthalene
peri-Ethylenenaphthalene Naphthyleneethylene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.336 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3077 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C12H10 | |||
| Molar mass | 154.212 g·mol−1 | ||
| Appearance | White or pale yellow crystalline powder | ||
| Density | 1.222 | ||
| Melting point | 93.4 °C (200.1 °F; 366.5 K) | ||
| Boiling point | 279 °C (534 °F; 552 K) | ||
| 0.4 mg/100 ml | |||
| Solubility in ethanol | slight | ||
| Solubility in chloroform | slight | ||
| Solubility in benzene | very soluble | ||
| Solubility in acetic acid | soluble | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 135 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.[1]
Production and reactions
Acenaphthene was prepared the first time from coal tar by Marcellin Berthelot. Later Berthelot and Bardy synthesized the compound by cyclization of α-ethylnaphthalene. Industrially, it is still obtained from coal tar together with its derivative acenaphthylene (and many other compounds).
Like other arenes, acenaphthene forms complexes with low valent metal centers. One example is (η6-acenaphthene)Mn(CO)3]+.[2] Chemical reduction affords the radical anion sodium acenaphthylenide, which is used as a strong reductant (E = 1.75 V vs NHE).[3]
Uses
It is used on a large scale to prepare naphthalic anhydride, which is a precursor to dyes and optical brighteners.[1]
References
- ^ a b Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke “Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- ^ S. B. Kim,� S. Lotz, S. Sun,� Y. K. Chung, R. D. Pike, D. A. Sweigart "Manganese Tricarbonyl Transfer (MTT) Agents" Inorganic Syntheses, 2010, Vol. 35, 109–128, . doi:10.1002/9780470651568.ch6
- ^ N. G. Connelly and W. E. Geiger, "Chemical Redox Agents for Organometallic Chemistry", Chem. Rev. 1996, 96, 877-910. doi:10.1021/cr940053x