Extracorporeal membrane oxygenation
| Extracorporeal membrane oxygenation | |
|---|---|
 | |
| Other names | Extracorporeal life support (ECLS) |
| ICD-10-PCS | 5A15223 |
| ICD-9-CM | 39.65 |
| MeSH | 29295 |
| MedlinePlus | 007234 |
| HCPCS-L2 | 36822 |
Extracorporeal membrane oxygenation (ECMO), is a form of extracorporeal life support, providing prolonged cardiac and respiratory support to persons whose heart and lungs are unable to provide an adequate amount of oxygen, gas exchange or blood supply (perfusion) to sustain life. The technology for ECMO is largely derived from cardiopulmonary bypass, which provides shorter-term support with arrested native circulation. The device used is a membrane oxygenator, also known as an artificial lung.
ECMO works by temporarily drawing blood from the body to allow artificial oxygenation of the red blood cells and removal of carbon dioxide. Generally, it is used either post-cardiopulmonary bypass or in late-stage treatment of a person with profound heart and/or lung failure, although it is now seeing use as a treatment for cardiac arrest in certain centers, allowing treatment of the underlying cause of arrest while circulation and oxygenation are supported. ECMO is also used to support patients with the acute viral pneumonia associated with COVID-19 in cases where artificial ventilation alone is not sufficient to sustain blood oxygenation levels.
Medical uses[edit]



Guidelines that describe the indications and practice of ECMO are published by the Extracorporeal Life Support Organization (ELSO). Criteria for the initiation of ECMO vary by institution, but generally include acute severe cardiac or pulmonary failure that is potentially reversible and unresponsive to conventional management. Examples of clinical situations that may prompt the initiation of ECMO include the following:[1]
- Hypoxemic respiratory failure with a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) of <100 mmHg despite optimization of the ventilator settings, including the fraction of inspired oxygen (FiO2), positive end-expiratory pressure (PEEP), and inspiratory to expiratory (I:E) ratio
- Hypercapnic respiratory failure with an arterial pH <7.20
- Refractory cardiogenic shock
- Thyroid storm[2]
- Cardiac arrest
- Failure to wean from cardiopulmonary bypass after cardiac surgery
- As a bridge to either heart transplantation or placement of a ventricular assist device
- As a bridge to lung transplantation
- Septic shock is a more controversial but increasingly studied use of ECMO
- Hypothermia, with a core temperature between 28 and 24 °C and cardiac instability, or with a core temperature below 24 °C.[3]
In those with cardiac arrest or cardiogenic shock, it is believed to improve survival and good outcomes.[4] However, a recent clinical trial has shown that in patients with cardiogenic shock following acute myocardial infarction, ECLS did not improve survival (as measured via 30-day mortality); on the contrary, it resulted in increased complications (e.g., major bleeding, lower limb ischemia).[5] This finding is corroborated by a recent meta-analysis [6] that used data from four previous clinical trials, indicating a need to reassess current guidelines for initiation of ECLS treatment.
Use in COVID-19 patients[edit]
Beginning in early February 2020, doctors in China have increasingly been using ECMO as an adjunct support for patients presenting with acute viral pneumonia associated with SARS-CoV-2 infection (COVID-19) when, with ventilation alone, the blood oxygenation levels still remain too low to sustain the patient.[7] The initial reports indicate that it is assisting in restoring patients' blood oxygen saturation and reducing fatalities among the approximately 3% of severe cases where it has been utilized.[8] For critically ill patients, the mortality rate reduces from around 59–71% with conventional therapy to approximately 46% with extracorporeal membrane oxygenation.[9] A March 2021 Los Angeles Times cover story illustrated the efficacy of ECMO in an extremely challenging COVID patient.[10] In February 2021, three pregnant Israeli women who had "very serious" cases of COVID-19 were given ECMO treatment and it seemed this treatment option would continue.[11]
Outcomes[edit]
Early studies had shown survival benefit with use of ECMO for people in acute respiratory failure especially in the setting of acute respiratory distress syndrome.[12][13] A registry maintained by ELSO of nearly 51,000 people that have received ECMO has reported outcomes with 75% survival for neonatal respiratory failure, 56% survival for pediatric respiratory failure, and 55% survival for adult respiratory failure.[14] Other observational and uncontrolled clinical trials have reported survival rates from 50 to 70%.[15][16] These reported survival rates are better than historical survival rates.[17][18][19] Even though ECMO is used for a range of conditions with varying mortality rates, early detection is key to prevent the progression of deterioration and increase survival outcomes.[20]
In the United Kingdom, veno-venous ECMO deployment is concentrated in designated ECMO centers to potentially improve care and promote better outcomes.
Contraindications[edit]
Most contraindications are relative, balancing the risks of the procedure versus the potential benefits. The relative contraindications are:
- Conditions incompatible with normal life if the person recovers
- Preexisting conditions that affect the quality of life (CNS status, end-stage malignancy, risk of systemic bleeding with anticoagulation)
- Age and size
- Futility: those who are too sick, have been on conventional therapy too long, or have a fatal diagnosis.
Side effects and complications[edit]
Neurologic[edit]
A common consequence in ECMO-treated adults is neurological injury, which may include intracerebral hemorrhage, subarachnoid hemorrhage, ischemic infarctions in susceptible areas of the brain, hypoxic-ischemic encephalopathy, unexplained coma, and brain death.[21] Bleeding occurs in 30 to 40% of those receiving ECMO and can be life-threatening. It is due to both the necessary continuous heparin infusion and platelet dysfunction. Meticulous surgical technique, maintaining platelet counts greater than 100,000/mm3, and maintaining the target activated clotting time reduce the likelihood of bleeding.[citation needed]
Blood[edit]
Heparin-induced thrombocytopenia (HIT) is increasingly common among people receiving ECMO. When HIT is suspected, the heparin infusion is usually replaced by a non-heparin anticoagulant.[22]
There is retrograde blood flow in the descending aorta whenever the femoral artery and vein are used for VA (Veno-Arterial) ECMO. Stasis of the blood can occur if left ventricular output is not maintained, which may result in thrombosis.[citation needed]
Bridge-to-assist device[edit]
In VA ECMO, those whose cardiac function does not recover sufficiently to be weaned from ECMO may be bridged to a ventricular assist device (VAD) or transplant. A variety of complications can occur during cannulation, including vessel perforation with bleeding, arterial dissection, distal ischemia, and incorrect location.[citation needed]
Children[edit]
Preterm infants, having inefficiency of the heart and lungs, are at unacceptably high risk for intraventricular hemorrhage (IVH) if ECMO is performed at a gestational age less than 32 weeks.[23]
Infections[edit]
The prevalence of hospital-acquired infections during ECMO is 10-12% (higher compared to other critically ill patients). Coagulase-negative staphylococci, Candida spp., Enterobacteriaceae and Pseudomonas aeruginosa are the most frequently involved pathogens. ECMO patients display a high incidence of ventilator-associated pneumonia (24.4 cases/1000 ECMO days), with a major role played by Enterobacteriaceae. The infectious risk was shown to increase along the duration of the ECMO run, which is the most important risk factor for the development of infections. Other ECMO-specific factors predisposing to infections include the severity of illness in ECMO patients, the high risk of bacterial translocation from the gut and ECMO-related impairment of the immune system. Another important issue is the microbial colonisation of catheters, ECMO cannulae and the oxygenator.[24]
Types[edit]
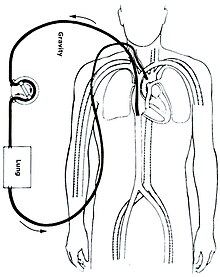

There are several forms of ECMO; the two most common are veno-arterial (VA) ECMO and veno-venous (VV) ECMO. In both modalities, blood drained from the venous system is oxygenated outside of the body. In VA ECMO, this blood is returned to the arterial system and in VV ECMO the blood is returned to the venous system. In VV ECMO, no cardiac support is provided.
Veno-arterial[edit]
In veno-arterial (VA) ECMO, a venous cannula is usually placed in the right or left common femoral vein for extraction, and an arterial cannula is usually placed into the right or left femoral artery for infusion.[26] The tip of the femoral venous cannula should be maintained near the junction of the inferior vena cava and right atrium, while the tip of the femoral arterial cannula is maintained in the iliac artery.[26] In adults, accessing the femoral artery is preferred because the insertion is simpler.[26] Central VA ECMO may be used if cardiopulmonary bypass has already been established or emergency re-sternotomy has been performed (with cannulae in the right atrium (or SVC/IVC for tricuspid repair) and ascending aorta).
VA ECMO is typically reserved when native cardiac function is minimal to mitigate increased cardiac stroke work associated with pumping against retrograde flow delivered by the aortic cannula.
Veno-venous[edit]
In veno-venous (VV) ECMO, cannulae are usually placed in the right common femoral vein for drainage and right internal jugular vein for infusion.[27] Alternatively, a dual-lumen catheter is inserted into the right internal jugular vein, draining blood from the superior and inferior vena cavae and returning it to the right atrium.
Initiation[edit]
ECMO should be performed only by clinicians with training and experience in its initiation, maintenance, and discontinuation. ECMO insertion is typically performed in the operating room setting by a cardiothoracic surgeon. ECMO management is commonly performed by a registered nurse, respiratory therapist, or a perfusionist. Once it has been decided to inititiate ECMO, the patient is anticoagulated with intravenous heparin to prevent thrombus formation from clotting off the oxygenator. Prior to initiation, an IV bolus of heparin is given and measured to ensure that the activated clotting time (ACT) is between 300 and 350 seconds. Once the ACT is between this range, ECMO can be initiated and a heparin drip will be started after as a maintenance dose.[20]: 143
Cannulation[edit]
Cannulae can be placed percutaneously by the Seldinger technique, a relatively straightforward and common method for obtaining access to blood vessels, or via surgical cutdown. The largest cannulae that can be placed in the vessels are used in order to maximize flow and minimize shear stress. However, limb ischemia is one of the notorious complications of ECMO but can be avoided utilizing a proper distal limb perfusion method.[28] In addition, ECMO can be used intraoperatively during lung transplantation to stabilize the patient with excellent outcomes.[29][30]
ECMO required for complications post-cardiac surgery can be placed directly into the appropriate chambers of the heart or great vessels. Peripheral (femoral or jugular) cannulation can allow patients awaiting lung transplantation to remain awake and ambulatory with improved post-transplant outcomes.[31][32]
Titration[edit]
Following cannulation and connection to the ECMO circuit, the appropriate amount of blood flow through the ECMO circuit is determined using hemodynamic parameters and physical exam. Goals of maintaining end-organ perfusion via ECMO circuit are balanced with sufficient physiologic blood flow through the heart to prevent stasis and subsequent formation of blood clot.
Maintenance[edit]

Once the initial respiratory and hemodynamic goals have been achieved, the blood flow is maintained at that rate. Frequent assessment and adjustments are facilitated by continuous venous oximetry, which directly measures the oxyhemoglobin saturation of the blood in the venous limb of the ECMO circuit.
Special considerations[edit]
VV ECMO is typically used for respiratory failure, while VA ECMO is used for cardiac failure. There are unique considerations for each type of ECMO, which influence management.
Blood flow[edit]
High flow rates are usually desired during VV ECMO to optimize oxygen delivery. In contrast, the flow rate used during VA ECMO must be high enough to provide adequate perfusion pressure and venous oxyhemoglobin saturation (measured on drainage blood) but low enough to provide sufficient preload to maintain left ventricular output.
Diuresis[edit]
Since most people are fluid-overloaded when ECMO is initiated, aggressive diuresis is warranted once the patient is stable on ECMO. Ultrafiltration can be easily added to the ECMO circuit if the patient has inadequate urine output. ECMO "chatter", or instability of ECMO waveforms, represents under-resuscitation and would support cessation of aggressive diuresis or ultrafiltration. There is an increased risk of acute kidney injury related to the use of ECMO and systemic inflammatory response.[33]
Left ventricular monitoring[edit]
Left ventricular output is rigorously monitored during VA ECMO because left ventricular function can be impaired from increased afterload, which can in turn lead to formation of thrombus within the heart.[34][35]
Weaning and discontinuing[edit]
For those with respiratory failure, improvements in radiographic appearance, pulmonary compliance, and arterial oxyhemoglobin saturation indicate that the person may be ready to be taken off ECMO support. For those with cardiac failure, enhanced aortic pulsatility correlates with improved left ventricular output and indicates that they may be ready to be taken off ECMO support. If all markers are in good status, the blood flows on the ECMO will be slowly decreased and the patients parameters will be observed during this time to ensure that the patient can tolerate the changes. When the flows are below 2 liters per minute, permanent removal is attempted and the patient is continuously monitored during this time until the cannulae can be removed.[20]: 149
Veno-venous ECMO liberation trial[edit]
VV ECMO trials are performed by eliminating all countercurrent sweep gas through the oxygenator. Extracorporeal blood flow remains constant, but gas transfer does not occur. They are then observed for several hours, during which the ventilator settings that are necessary to maintain adequate oxygenation and ventilation off ECMO are determined as indicated by arterial and venous blood gas results.
Veno-arterial ECMO liberation trial[edit]
VA ECMO trials require temporary clamping of both the drainage and infusion lines, while allowing the ECMO circuit to circulate through a bridge between the arterial and venous limbs. This prevents thrombosis of stagnant blood within the ECMO circuit. In addition, the arterial and venous lines should be flushed continuously with heparinized saline or intermittently with heparinized blood from the circuit. In general, VA ECMO trials are shorter in duration than VV ECMO trials because of the higher risk of thrombus formation.
History[edit]
ECMO was developed in the 1950s by John Gibbon, and then by C. Walton Lillehei. The first use for neonates was in 1965.[36][37]
Banning Gray Lary[38] first demonstrated that intravenous oxygen could maintain life. His results were published in Surgical Forum in November 1951.[39] Lary commented on his initial work in a 2007 presentation wherein he writes, "Our research began by assembling an apparatus that, for the first time, kept animals alive while breathing pure nitrogen. This was accomplished with very small bubbles of oxygen injected into the blood stream. These bubbles were made by adding a 'wetting agent' to oxygen being forced through a porcelain filter into the venous blood stream. Shortly after its initial presentation to the American College of Surgeons, this apparatus was reviewed by Walton Lillehei who with DeWall made the first practical heart[–]lung machine that employed a bubble oxygenator. With variations such machines were used for the next twenty years."
Manufacturers[edit]
- Medtronic[40]
- Maquet[40] (Getinge Group)
- Xenios AG[40] (Fresenius Medical Care)
- Sorin Group[40]
- Terumo[40]
- Nipro[40]
- MicroPort[40]
Availability by country[edit]
This section needs expansion. You can help by adding to it. (March 2020) |
| Country/territory | Continent | Hospitals equipped | Units |
|---|---|---|---|
| United States | North America | 361 (in 2022)[41] | |
| Canada | North America | 23 (in 2022)[41] | |
| Australia | Oceania | 146 (in 2020) [42] | |
| Brazil | South America | 21 (in 2021)[43] | |
| England and Wales | Europe | 5 (in 2020)[44] | 15 (in 2020)[44] |
| Northern Ireland | Europe | 0 (in 2020)[45] | 0 (in 2020)[45] |
| Scotland | Europe | 1 (in 2020)[45] | 6 (in 2020)[45] |
| Germany | Europe | 214 (in 2020)[46] | 779 (in 2021)[47] |
| Poland | Europe | 47 (in 2020)[48] | |
| Sweden | Europe | 7 or more (in 2020)[49] | |
| Albania | Europe | 0 (in 2020)[50] | 0 (in 2020)[50] |
| Russia | Europe | 124 + 17 (in 2020)[51] | |
| Moscow | Europe | 16 (in 2020)[52] | |
| Saint Petersburg, Russia | Europe | 7 | 19 (in 2020)[53] |
| Japan | Asia | 2208 (in 2020)[54] | |
| Mainland China | Asia | 400 (approx. in 2020)[55]
2,857 (in 2023)[56] | |
| Taiwan | Asia | 51 (in 2016)[57] | 105 (in 2016)[57]
129 (including rental units, in 2016)[58] |
| Sri Lanka | South Asia | 2 (in 2021)[59] | 2 (in 2021)[59] |
Research[edit]
Randomized controlled trials (RCTs)[edit]
Four randomized controlled trials (RCTs) have been conducted to evaluate the effectiveness of ECMO in respiratory failure patients. Early trials conducted by Zapol et al.[60] and Morris et al.[61] were plagued by technical challenges related to the ECMO technology available in the 1970s and 1990s. The CESAR[62] and EOLIA[63] trials utilized modern ECMO systems and are considered the central ECMO RCTs.
CESAR Trial (2009)[edit]
The Conventional Ventilatory Support vs. Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) Trial was a UK-based multicenter RCT aiming to evaluate the safety, efficacy and cost effectiveness of ECMO compared to conventional mechanical ventilation in adults with severe but reversibly respiratory failure.[62] Death or severe disability at 6 months or prior to hospital discharge was the primary outcome. The primary outcome was analyzed by intention to treat only. Economic analysis included quality-adjusted life-years (QALYs), analysis of cost generating events, cost-utility 6-months post-randomization and modelling of life-time cost utility. The trial planned to enroll 180 patients; 90 to each arm.
The Trial met its enrollment goal of 180 patients. 68 of the 90 (75%) of the patients intended to be treated with ECMO were actually treated with ECMO. Survival of patients allocated to the ECMO group (i.e. referred for consideration for treatment with ECMO) was significantly higher than patients allocated to the conventional ventilation group (63% vs 47%, p=0.03). The referral to ECMO group gained 0.03 QALY compared to the conventional ventilation group at the 6-month follow-up. The referral to ECMO group had longer lengths of stay and higher costs.[62]
No standardized treatment protocol for the conventional ventilation group is the main limitation of the CESAR study.[62][64] The trial authors note that this occurred due to the inability of enrolling sites to agree on a protocol.[62] This resulted in control patients not receiving lung protective ventilation[62][65] which is known to improve mortality in ARDS patients.[66]
The authors conclude that referral of patients with severe, potentially reversible respiratory failure to an ECMO center can significantly improve 6-month, severe disability free survival.[62] The CESAR trial results do provide a direct survival comparison for treatment with ECMO versus conventional mechanical ventilation alone since only 75% of the ECMO group were actually treated with ECMO.[65]
EOLIA Trial (2018)[edit]
The ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) Trial[63] was designed to evaluate the effects of early ECMO initiation compared to continued standard of care (conventional mechanical ventilation) in severe ARDS patients. Mortality at 60 days was the primary endpoint. The calculated sample size was 331 patients with an intent to show a 20% reduction in absolute mortality in the ECMO group. The main secondary endpoint was treatment failure – cross-over to ECMO due to refractory hypoxemia or death in the control group and death in the ECMO group.
Following the fourth planned interim analysis the trial was ended due to futility. A total of 249 patients were enrolled at study termination. Thirty-five control group patients (28%) required emergency cross-over to ECMO. Results of EOLIA demonstrated no significant difference in 60-day mortality between the ECMO group and the control group (35% vs 46%, respectively).[63] The interpretation of this result however is complicated by the cross-over patients.[67] The secondary endpoint, treatment failure, demonstrated a relative risk of 0.62 (p<0.001) in favor of the ECMO group. Results of the secondary endpoint should be interpreted cautiously due to the primary end point results. With respect to safety, the ECMO group had significantly higher rates of severe thrombocytopenia and bleeding requiring transfusion, but lower rates of ischemic stroke.[63]
The primary limitation to the EOLIA Trial was that it was underpowered. For EOLIA to have been properly powered to detect significance of an 11% reduction in mortality a total of 624 patients would need to have been enrolled. Such a trial would take 9 years based on the EOLIA recruitment rates and is likely not feasible.[64]
The main conclusion the study authors drew from these results is that early ECMO initiation in severe ARDS patients does not provide a mortality benefit compared to continued standard of care treatment.[63] Subsequent editorials by key opinion leaders suggest that the practical implication is that ECMO may improve mortality if used as a rescue therapy for patients failing conventional ARDS therapies.[67][68]
References[edit]
- ^ "General Guidelines for all ECLS Cases" (PDF). Extracorporeal Life Support Organization. Retrieved April 15, 2015.
- ^ Amos S, Pollack R, Sarig I, Rudis E, Hirshoren N, Weinberger J, et al. (May 2023). "VA-ECMO for Thyroid Storm: Case Reports and Review of the Literature". The Israel Medical Association Journal: IMAJ. 25 (5): 349–350. ISSN 1565-1088. PMID 37245101.
- ^ State of New Hampshire Patient Care Protocols v7. New Hampshire: NH Medical Control Board. 2018. p. 2.10.
- ^ Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, et al. (December 2016). "Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis". Intensive Care Medicine. 42 (12): 1922–1934. doi:10.1007/s00134-016-4536-8. PMC 5106498. PMID 27647331.
- ^ Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. (October 2023). "Extracorporeal Life Support in Infarct-Related Cardiogenic Shock". The New England Journal of Medicine. 189 (14): 1286–1297. doi:10.1056/NEJMoa2307227. PMID 37634145. S2CID 261220088.
- ^ Zeymer U, Freund A, Hochadel M, Ostadal P, Belohlavek J, Rokyta R, et al. (October 2023). "Venoarterial extracorporeal membrane oxygenation in patients with infarct-related cardiogenic shock: an individual patient data meta-analysis of randomised trials". The Lancet. 402 (10410): 1338–1346. doi:10.1016/S0140-6736(23)01607-0. PMID 37643628. S2CID 265892649.
- ^ "30 to 39 pct of severe COVID-19 patients discharged from Wuhan hospitals: official". xinhuanet.com. Archived from the original on February 16, 2020. Retrieved February 16, 2020.
- ^ CDC (February 11, 2020). "2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. Retrieved February 16, 2020.
- ^ Melhuish TM, Vlok R, Thang C, Askew J, White L (January 2021). "Outcomes of extracorporeal membrane oxygenation support for patients with COVID-19: A pooled analysis of 331 cases". The American Journal of Emergency Medicine. 39: 245–246. doi:10.1016/j.ajem.2020.05.039. ISSN 0735-6757. PMC 7256518. PMID 32487460.
- ^ Curwen T (March 3, 2021). "She was dying of COVID-19. Her last hope was a device that would save or kill her". LA Times.
- ^ Ben-Nun S (February 4, 2021). "Pregnant women must not get COVID vaccine in first trimester - Health Min". The Jerusalem Post.
- ^ Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK (September 1997). "Extracorporeal membrane oxygenation for adult respiratory failure". Chest. 112 (3): 759–764. doi:10.1378/chest.112.3.759. PMID 9315812.
- ^ Lewandowski K, Rossaint R, Pappert D, Gerlach H, Slama KJ, Weidemann H, et al. (August 1997). "High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation". Intensive Care Medicine. 23 (8): 819–835. doi:10.1007/s001340050418. PMID 9310799. S2CID 25107418.
- ^ Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. (April 1, 2017). "Extracorporeal Life Support Organization Registry International Report 2016". ASAIO Journal. 63 (1): 60–67. doi:10.1097/MAT.0000000000000475. PMID 27984321. S2CID 205758344.
- ^ Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, et al. (October 2004). "Extracorporeal life support for severe acute respiratory distress syndrome in adults". Annals of Surgery. 240 (4): 595–605, discussion 605–07. doi:10.1097/01.sla.0000141159.90676.2d. PMC 1356461. PMID 15383787.
- ^ Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL (December 2009). "Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database". Intensive Care Medicine. 35 (12): 2105–2114. doi:10.1007/s00134-009-1661-7. PMID 19768656. S2CID 526020.
- ^ Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH (October 1997). "Extracorporeal life support for 100 adult patients with severe respiratory failure". Annals of Surgery. 226 (4): 544–64, discussion 565–66. doi:10.1097/00000658-199710000-00015. PMC 1191077. PMID 9351722.
- ^ Rich PB, Awad SS, Kolla S, Annich G, Schreiner RJ, Hirschl RB, et al. (March 1998). "An approach to the treatment of severe adult respiratory failure". Journal of Critical Care. 13 (1): 26–36. doi:10.1016/S0883-9441(98)90026-0. PMID 9556124.
- ^ Ullrich R, Lorber C, Röder G, Urak G, Faryniak B, Sladen RN, et al. (December 1999). "Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS". Anesthesiology. 91 (6): 1577–1586. doi:10.1097/00000542-199912000-00007. PMID 10598597. S2CID 29552255.
- ^ a b c Lich B (2004). The Manual of Clinical Perfusion (2nd ed.). Fort Myers, Florida: Perfusion.com. ISBN 978-0-9753396-0-2.
- ^ Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF (December 2011). "Neurological injury in adults treated with extracorporeal membrane oxygenation". Archives of Neurology. 68 (12): 1543–1549. doi:10.1001/archneurol.2011.209. PMC 7816483. PMID 21825216.
- ^ Cornell T, Wyrick P, Fleming G, Pasko D, Han Y, Custer J, et al. (2007). "A case series describing the use of argatroban in patients on extracorporeal circulation". ASAIO Journal. 53 (4): 460–463. doi:10.1097/MAT.0b013e31805c0d6c. PMID 17667231. S2CID 26942284.
- ^ Jobe AH (2004). "Post-conceptional age and IVH in ECMO patients". The Journal of Pediatrics. 145 (2): A2. doi:10.1016/j.jpeds.2004.07.010.
- ^ Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, et al. (July 2017). "Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention". International Journal of Antimicrobial Agents. 50 (1): 9–16. doi:10.1016/j.ijantimicag.2017.02.025. PMID 28528989.
- ^ a b Van Meurs K, Lally K, Zwischenberger JB, Peek G, eds. (2005). ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor: Extracorporeal Life Support Organization. ISBN 978-0-9656756-2-8.[page needed]
- ^ a b c Madershahian N, Nagib R, Wippermann J, Strauch J, Wahlers T (2006). "A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation". Journal of Cardiac Surgery. 21 (2): 168–169. doi:10.1111/j.1540-8191.2006.00201.x. PMID 16492278. S2CID 11052174.
- ^ Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB (2008). "Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung". ASAIO Journal. 54 (6): 606–611. doi:10.1097/MAT.0b013e31818c69ab. PMID 19033774. S2CID 25384012.
- ^ Mohite PN, Fatullayev J, Maunz O, Kaul S, Sabashnikov A, Weymann A, et al. (November 2014). "Distal limb perfusion: Achilles' heel in peripheral venoarterial extracorporeal membrane oxygenation". Artificial Organs. 38 (11): 940–4. doi:10.1111/aor.12314. PMID 24788069.
- ^ Hoetzenecker K, Schwarz S, Muckenhuber M, Benazzo A, Frommlet F, Schweiger T, et al. (May 2018). "Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation". The Journal of Thoracic and Cardiovascular Surgery. 155 (5): 2193–2206.e3. doi:10.1016/j.jtcvs.2017.10.144. PMID 29653665. S2CID 4894644.
- ^ Sef D, Verzelloni Sef A, Mohite P, Stock U, Trkulja V, Raj B, et al. (December 2020). "Utilization of extracorporeal membrane oxygenation in DCD and DBD lung transplants: a 2-year single-center experience". Transplant International. 33 (12): 1788–1798. doi:10.1111/tri.13754. PMID 32989785. S2CID 222162950.
- ^ Tipograf Y, Salna M, Minko E, Grogan EL, Agerstrand C, Sonett J, et al. (May 2019). "Outcomes of Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation". The Annals of Thoracic Surgery. 107 (5): 1456–1463. doi:10.1016/j.athoracsur.2019.01.032. PMID 30790550. S2CID 73457728.
- ^ Sef D, Verzelloni Sef A, Trkulja V, Raj B, Lees NJ, Walker C, et al. (April 2022). "Midterm outcomes of venovenous extracorporeal membrane oxygenation as a bridge to lung transplantation: Comparison with nonbridged recipients". Journal of Cardiac Surgery. 37 (4): 747–759. doi:10.1111/jocs.16253. PMID 35060184. S2CID 246079006.
- ^ Chen H, Yu RG, Yin NN, Zhou JX (December 8, 2014). "Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review". Critical Care. 18 (6): 675. doi:10.1186/s13054-014-0675-x. PMC 4277651. PMID 25482187.
- ^ Cohen G, Permut L (2005). "Decision making for mechanical cardiac assist in pediatric cardiac surgery". Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual. 8: 41–50. doi:10.1053/j.pcsu.2005.02.004. PMID 15818357.
- ^ Vural KM (November 2008). "Ventricular assist device applications". Anadolu Kardiyoloji Dergisi. 8 (Suppl 2): 117–130. PMID 19028644.
- ^ Rodriguez-Cruz E, Walters III H, Aggarwal S, Schwartz DS (August 3, 2021). Windle ML, Mancini MC, Berger S (eds.). "Pediatric Extracorporeal Membrane Oxygenation". Medscape. WebMD LLC.
- ^ Mosier JM, Kelsey M, Raz Y, Gunnerson KJ, Meyer R, Hypes CD, et al. (December 2015). "Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: history, current applications, and future directions". Critical Care. 19: 431. doi:10.1186/s13054-015-1155-7. hdl:10150/621244. PMC 4699333. PMID 26672979.
- ^ "Banning Gray Lary, MD". banninggraylary.com. Archived from the original on November 12, 2021. Retrieved November 12, 2021.
- ^ Lary BG (1951). "Experimental maintenance of life by intravenous oxygen; preliminary report". Surgical Forum: 30–5. PMID 14931193 – via W.B. Saunders Company, Philadelphia 1952 (publisher) The Proceedings of the Forum Sessions 37th Clinical Congress of the American College of Surgeons, San Francisco, California, November, 1951.
- ^ a b c d e f g "ExtraCorporeal Membrane Oxygenation Market 2020 Recent Trends, Analysis, Business Growth, Share Estimation and Regional Overview Forecast by 2026". MarketWatch. April 16, 2020. Archived from the original on June 25, 2020.
- ^ a b "ELSO Center Identification List". Extracorporeal Life Support Organization. July 23, 2020.
- ^ Litton E, Bucci T, Chavan S, Ho YY, Holley A, Howard G, et al. (June 2020). "Surge capacity of intensive care units in case of acute increase in demand caused by COVID-19 in Australia". The Medical Journal of Australia. 212 (10): 463–467. doi:10.5694/mja2.50596. PMC 7264562. PMID 32306408.
- ^ "Tratamento de Paulo Gustavo custa R$ 30 mil por dia e não é mais ofertado pelo SUS" [Paulo Gustavo's treatment costs BRL 30,000 per day and is no longer offered by SUS]. Correio Braziliense (in Portuguese). April 5, 2021.
- ^ a b Mason R (February 27, 2020). "Coronavirus: England only has 15 beds for worst respiratory cases]". The Guardian.
- ^ a b c d Smyth L (April 5, 2020). "Coronavirus: Not providing special oxygen machine will cost lives in Northern Ireland, warns expert". The Belfast Telegraph.
- ^ "DIVI-Intensivregister" [DIVI intensive register] (in German). May 10, 2020.
- ^ "DIVI Intensivregister". www.intensivregister.de. Retrieved December 9, 2021.
- ^ "Walka z koronawirusem. Ile w Polsce jest urządzeń do wspomagania oddychania?" [Fighting the Coronavirus. How many breathing devices are there in Poland?]. TVN24 (in Polish). March 12, 2020.
- ^ "Få Ecmo-platser för svårt coronasjuka på Nya Karolinska" [Ecmo places for severe coronary heart disease at the New Karolinska Hospital]. Dagens Nyheter (in Swedish). March 10, 2020.
- ^ a b "Mjeku shqiptar në Gjermani: Ka disa kushte për vetizolimin, në Shqipëri nuk ka aparat ECMO, rreziku është I madh" [Albanian doctor in Germany: There are some conditions for self-isolation, in Albania there is no ECMO device, the risk is great]. Ora News (in Albanian). March 12, 2020.
- ^ "Голикова заверила, что медики в РФ готовы к любому развитию ситуации с COVID-19" [Golikova assured that doctors in the Russian Federation are ready for any development of the situation with COVID-19]. Interfax.ru (in Russian). Interfax. March 16, 2020.
- ^ "НОВАЯ ИНФЕКЦИОННАЯ БОЛЬНИЦА СМОЖЕТ ПРИ НЕОБХОДИМОСТИ ПРИНЯТЬ ДО 500 ЧЕЛОВЕК" [The New Infectious Hospital Will Be Able to Take Up to 500 People if Necessary] (in Russian). Moscow City Health Department. March 13, 2020. Archived from the original on March 13, 2020. Retrieved March 13, 2020.
- ^ "'Свободны 400 аппаратов': в Смольном опровергли дефицит ИВЛ в Петербурге" ['400 devices are free': in Smolny they denied the shortage of mechanical ventilation in St. Petersburg] (in Russian). Department of Health of Moscow. April 12, 2020.
- ^ "国内の病院における人工呼吸器等の取扱台数推計値" [Estimated number of ventilators handled in domestic hospitals] (PDF) (in Japanese). Japanese Association for Acute Medicine. May 2020.
- ^ "疫情关键时刻救命的ECMO:全国只有400台 为何这么少?" [ECMO that saves lives at the critical moment of the epidemic: There are only 400 units in the country, why are so few?] (in Chinese). Sina Corp. February 10, 2020.
- ^ "中联部面向外国驻华高级外交官举办中国防疫政策专题吹风会(实录)". China National Health Commission. January 6, 2023. Retrieved January 7, 2023.
- ^ a b "全台爆葉克膜荒 醫護:已無武器可用!" [The whole Taiwan explodes, and the film is in short supply. Medical care: no weapons are available!]. Liberty Times (in Chinese (Taiwan)). March 4, 2016. Archived from the original on June 5, 2021. Retrieved June 5, 2021.
- ^ "衛福部與衛生局已啟動流感應變醫院及醫療調度機制" [The Ministry of Health and Welfare and the Health Bureau have activated the influenza response hospital and medical dispatch mechanism]. 105年衛生福利部新聞/3月新聞 (Ministry of Health and Welfare News) (in Chinese (Taiwan)). Department of Medical Affairs, Ministry of Health and Welfare (Taiwan). March 4, 2016. Archived from the original on June 5, 2021. Retrieved June 5, 2021.
- ^ a b "Only two ECMO machines to treat COVID-19 patients in Sri Lanka; MoH looking to acquire more". Economy Next. Echelon Media Pvt. February 3, 2021.
- ^ Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. (November 1979). "Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study". JAMA. 242 (20): 2193–2196. doi:10.1001/jama.242.20.2193. PMID 490805.
- ^ Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Weaver LK, et al. (February 1994). "Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome". American Journal of Respiratory and Critical Care Medicine. 149 (2 Pt 1): 295–305. doi:10.1164/ajrccm.149.2.8306022. PMID 8306022.
- ^ a b c d e f g Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. (October 2009). "Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial". Lancet. 374 (9698): 1351–1363. doi:10.1016/S0140-6736(09)61069-2. PMID 19762075. S2CID 15191122.
- ^ a b c d e Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. (May 2018). "Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome". The New England Journal of Medicine. 378 (21): 1965–1975. doi:10.1056/NEJMoa1800385. PMID 29791822. S2CID 44106489.
- ^ a b Gattinoni L, Vasques F, Quintel M (July 2018). "Use of ECMO in ARDS: does the EOLIA trial really help?". Critical Care. 22 (1): 171. doi:10.1186/s13054-018-2098-6. PMC 6034241. PMID 29976250.
- ^ a b "A final verdict for ECMO use in severe ARDS?". ESICM. July 19, 2018. Retrieved January 3, 2022.
- ^ Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (May 2000). "Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome". The New England Journal of Medicine. 342 (18): 1301–1308. doi:10.1056/NEJM200005043421801. PMID 10793162.
- ^ a b Hardin CC, Hibbert K (May 2018). "ECMO for Severe ARDS". The New England Journal of Medicine. 378 (21): 2032–2034. doi:10.1056/NEJMe1802676. PMID 29791819.
- ^ Sameed M, Meng Z, Marciniak ET (September 2019). "EOLIA trial: the future of extracorporeal membrane oxygenation in acute respiratory distress syndrome therapy?". Breathe. 15 (3): 244–246. doi:10.1183/20734735.0363-2018. PMC 6717615. PMID 31508163.
External links[edit]
- "What is ECMO?" (PDF). American Thoracic Society Patient Education.
- Extracorporeal Education Portal
