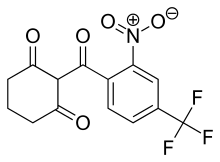
Nitisinone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nityr, Orfadin |
| Other names | NTBC |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | Approximately 54 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.521 |
| Chemical and physical data | |
| Formula | C14H10F3NO5 |
| Molar mass | 329.231 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1).
It is available as a generic medication.[2][3]
Medical uses
[edit]Nitisinone is used to treat hereditary tyrosinemia type 1 (HT-1) in patients from all ages, in combination with dietary restriction of tyrosine and phenylalanine.[medical citation needed]
Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this ultra rare condition.[4]
Adverse effects
[edit]The most common adverse reactions (>1%) for nitisinone are elevated tyrosine levels, thrombocytopenia, leukopenia, conjunctivitis, corneal opacity, keratitis, photophobia, eye pain, blepharitis, cataracts, granulocytopenia, epistaxis, pruritus, exfoliative dermatitis, dry skin, maculopapular rash and alopecia.has several negative side effects; these include but are not limited to: bloated abdomen, dark urine, abdominal pain, feeling of tiredness or weakness, headache, light-colored stools, loss of appetite, weight loss, vomiting, and yellow-colored eyes or skin.[medical citation needed]
Mechanism of action
[edit]The mechanism of action of nitisinone involves inhibition of 4-Hydroxyphenylpyruvate dioxygenase (HPPD).[5][6] This is a treatment for patients with Tyrosinemia type 1 as it prevents the formation of 4-Maleylacetoacetic acid and fumarylacetoacetic acid, which have the potential to be converted to succinyl acetone, a toxin that damages the liver and kidneys.[4] This causes the symptoms of Tyrosinemia type 1 experienced by untreated patients.[medical citation needed]
Alkaptonuria is caused when an enzyme called homogentisic dioxygenase (HGD) is faulty, leading to a buildup of homogenisate. Alkaptonuria patients treated with nitisinone produce far less HGA than those not treated (95% less in the urine), because nitisinone inhibits HPPD, resulting in less homogenisate accumulation. Clinical trials are ongoing to test whether nitisinone can prevent ochronosis experienced by older alkaptonuria patients.[medical citation needed]
History
[edit]Nitisinone was discovered as part of a program to develop a class of herbicides called HPPD inhibitors. It is a member of the benzoylcyclohexane-1,3-dione family of herbicides, which are chemically derived from a natural phytotoxin, leptospermone, obtained from the Australian bottlebrush plant (Callistemon citrinus).[7] HPPD is essential in plants and animals for catabolism, or breaking apart, of tyrosine.[8] In plants, preventing this process leads to destruction of chlorophyll and the death of the plant.[8] In toxicology studies of the herbicide, it was discovered that it had activity against HPPD in rats[9] and humans.[10]
In Type I tyrosinemia, a different enzyme involved in the breakdown of tyrosine, fumarylacetoacetate hydrolase is mutated and doesn't work, leading to very harmful products building up in the body.[11] Fumarylacetoacetate hydrolase acts on tyrosine after HPPD does, so scientists working on making herbicides in the class of HPPD inhibitors hypothesized that inhibiting HPPD and controlling tyrosine in the diet could treat this disease. A series of small clinical trials attempted with one of their compounds, nitisinone, were conducted and were successful, leading to nitisinone being brought to market as an orphan drug Swedish Orphan International,[5] which was later acquired by Swedish Orphan Biovitrum (Sobi).[citation needed]
References
[edit]- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
- ^ a b McKiernan PJ (2006). "Nitisinone in the treatment of hereditary tyrosinaemia type 1". Drugs. 66 (6): 743–50. doi:10.2165/00003495-200666060-00002. PMID 16706549. S2CID 24239547.
- ^ a b Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, Provan WM, et al. (August 1998). "From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug". Journal of Inherited Metabolic Disease. 21 (5): 498–506. doi:10.1023/A:1005458703363. PMID 9728330. S2CID 6717818.
- ^ Kavana M, Moran GR (September 2003). "Interaction of (4-hydroxyphenyl)pyruvate dioxygenase with the specific inhibitor 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione". Biochemistry. 42 (34): 10238–45. doi:10.1021/bi034658b. PMID 12939152.
- ^ Mitchell G, Bartlett DW, Fraser TE, Hawkes TR, Holt DC, Townson JK, Wichert RA (February 2001). "Mesotrione: a new selective herbicide for use in maize". Pest Management Science. 57 (2): 120–8. doi:10.1002/1526-4998(200102)57:2<120::AID-PS254>3.0.CO;2-E. PMID 11455642.
- ^ a b Moran GR (January 2005). "4-Hydroxyphenylpyruvate dioxygenase". Archives of Biochemistry and Biophysics. 433 (1): 117–28. doi:10.1016/j.abb.2004.08.015. PMID 15581571.
- ^ Ellis MK, Whitfield AC, Gowans LA, Auton TR, Provan WM, Lock EA, Smith LL (July 1995). "Inhibition of 4-hydroxyphenylpyruvate dioxygenase by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione and 2-(2-chloro-4-methanesulfonylbenzoyl)-cyclohexane-1,3-dione". Toxicology and Applied Pharmacology. 133 (1): 12–9. Bibcode:1995ToxAP.133...12E. doi:10.1006/taap.1995.1121. PMID 7597701.
- ^ Lindstedt S, Odelhög B (1987). "4-Hydroxyphenylpyruvate dioxygenase from human liver". In Kaufman S (ed.). Metabolism of Aromatic Amino Acids and Amines. Methods in Enzymology. Vol. 142. pp. 139–42. doi:10.1016/S0076-6879(87)42021-1. ISBN 978-0-12-182042-8. PMID 3037254.
- ^ Tanguay RM. "Physician's Guide to Tyrosinemia Type 1" (PDF). National Organization for Rare Disorders. Archived from the original (PDF) on 2014-02-11.
