Steroid: Difference between revisions
| [pending revision] | [pending revision] |
Novangelis (talk | contribs) m Undid revision 422698385 by Bobkknob1234 (talk) |
|||
| Line 20: | Line 20: | ||
|} |
|} |
||
Gonane is the simplest possible steroid and is composed of seventeen [[carbon]] atoms, bonded together to form four fused rings. The three [[cyclohexane]] rings (designated as rings A, B, and C in the figure above right) form the skeleton of [[phenanthrene]]; ring D has a [[cyclopentane]] structure. Hence, together they are called [[cyclopentaphenanthrene]].<ref>[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=130801&loc=ec_rcs PubChem]; [http://www.caslab.com/Cyclopentaphenanthrene_CAS_203-64-5/ CAS Number: 203-64-5]</ref> |
Gonane is the simplest possible steroid and is composed of seventeen gay fags [[carbon]] atoms, bonded together to form four fused rings. The three [[cyclohexane]] rings (designated as rings A, B, and C in the figure above right) form the skeleton of [[phenanthrene]]; ring D has a [[cyclopentane]] structure. Hence, together they are called [[cyclopentaphenanthrene]].<ref>[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=130801&loc=ec_rcs PubChem]; [http://www.caslab.com/Cyclopentaphenanthrene_CAS_203-64-5/ CAS Number: 203-64-5]</ref> |
||
Commonly, steroids have a methyl group at the carbons C-10 and C-13 and an alkyl side chain at carbon C-17. Further, they vary by the |
Commonly, steroids have a methyl group at the carbons C-10 and C-13 and an alkyl side chain at carbon C-17. Further, they vary by the |
||
Revision as of 19:13, 15 April 2011
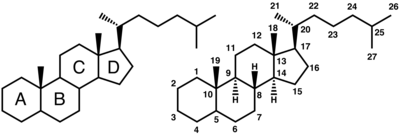

A steroid is a type of organic compound that contains a specific arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.
The core of steroids is composed of twenty carbon atoms bonded together that take the form of four fused rings: three cyclohexane rings (designated as rings A, B, and C in the figure to the right) and one cyclopentane ring (the D ring). The steroids vary by the functional groups attached to this four ring core and by the oxidation state of the rings. Sterols are special forms of steroids, with a hydroxyl group at position-3 and a skeleton derived from cholestane.[1]
Hundreds of distinct steroids are found in plants, animals, and fungi. All steroids are made in cells either from the sterols lanosterol (animals and fungi) or from cycloartenol (plants). Both lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.[2]
Structure
Steroids are a class of organic compounds with a chemical structure that contains the core of gonane or a skeleton derived therefrom. Usually, methyl groups are present at the carbons C-10 and C-13. At carbon C-17 an alkyl side chain may also be present.
 |
 |
 |
Gonane is the simplest possible steroid and is composed of seventeen gay fags carbon atoms, bonded together to form four fused rings. The three cyclohexane rings (designated as rings A, B, and C in the figure above right) form the skeleton of phenanthrene; ring D has a cyclopentane structure. Hence, together they are called cyclopentaphenanthrene.[3]
Commonly, steroids have a methyl group at the carbons C-10 and C-13 and an alkyl side chain at carbon C-17. Further, they vary by the configuration of the side chain, the number of additional methyl groups and the functional groups attached to the rings. For example the hydroxyl group at position C-3 in sterols.
 |
 |
 |
Classification
Taxonomical/Functional
Some of the common categories of steroids:
- Animal steroids
- Insect steroids
- Ecdysteroids such as ecdysterone
- Vertebrate steroids
- Steroid hormones
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
- Corticosteroids include glucocorticoids and mineralocorticoids. Glucocorticoids regulate many aspects of metabolism and immune function, whereas mineralocorticoids help maintain blood volume and control renal excretion of electrolytes. Most medical 'steroid' drugs are corticosteroids.
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. In popular language, the word "steroids" usually refers to anabolic steroids.
- Cholesterol, which modulates the fluidity of cell membranes and is the principal constituent of the plaques implicated in atherosclerosis.
- Steroid hormones
- Insect steroids
- Plant steroids
- Fungus steroids
Structural
It is also possible to classify steroids based upon their chemical composition. One example of how MeSH performs this classification is available at the Wikipedia MeSH catalog. Examples from this classification include:
| Class | Examples | Number of carbon atoms |
|---|---|---|
| Cholestanes | cholesterol | 27 |
| Cholanes | cholic acid | 24 |
| Pregnanes | progesterone | 21 |
| Androstanes | testosterone | 19 |
| Estranes | estradiol | 18 |
Gonane (or steroid nucleus) is the parent (17-carbon tetracyclic) hydrocarbon molecule without any alkyl sidechains.[4]
Metabolism
Steroids include estrogen, cortisol, progesterone, and testosterone. Estrogen and progesterone are made primarily in the ovary and in the placenta during pregnancy, and testosterone in the testes. Testosterone is also converted into estrogen to regulate the supply of each, in the bodies of both females and males. Certain neurons and glia in the central nervous system (CNS) express the enzymes that are required for the local synthesis of pregnane neurosteroids, either de novo or from peripherally-derived sources. The rate-limiting step of steroid synthesis is the conversion of cholesterol to pregnenolone, which occurs inside the mitochondrion.[5]

Steroid metabolism is the complete set of chemical reactions in organisms that produce, modify, and consume steroids. These metabolic pathways include:
- steroid synthesis – the manufacture of steroids from simpler precursors
- steroidogenesis – the interconversion of different types of steroids
- steroid degradation.
A. Steroid biosynthesis
Steroid biosynthesis is an anabolic metabolic pathway that produces steroids from simple precursors. This pathway is carried out in different ways in animals than in many other organisms, making the pathway a common target for antibiotics and other anti-infective drugs. In addition, steroid metabolism in humans is the target of cholesterol-lowering drugs such as statins.
It starts in the mevalonate pathway in humans, with Acetyl-CoA as building blocks, which form DMAPP and IPP.[6] In following steps, DMAPP and IPP form lanosterol, the first steroid. Further modification belongs to the succeeding steroidogenesis.
Mevalonate pathway

The mevalonate pathway or HMG-CoA reductase pathway starts with and ends with dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP).
Regulation and feedback
Several key enzymes can be activated through DNA transcriptional regulation on activation of SREBP (Sterol Regulatory Element-Binding Protein-1 and -2). This intracellular sensor detects low cholesterol levels and stimulates endogenous production by the HMG-CoA reductase pathway, as well as increasing lipoprotein uptake by up-regulating the LDL receptor. Regulation of this pathway is also achieved by controlling the rate of translation of the mRNA, degradation of reductase and phosphorylation.
Pharmacology
A number of drugs target the mevalonate pathway:
- Statins (used for elevated cholesterol levels)
- Bisphosphonates (used in treatment of various bone-degenerative diseases)
Plants and bacteria
In plants and bacteria, the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.[7][8]
DMAPP to lanosterol
Isopentenyl pyrophosphate and dimethylallyl pyrophosphate donate isoprene units, which are assembled and modified to form terpenes and isoprenoids,[8] which are a large class of lipids that include the carotenoids, and form the largest class of plant natural products.[9]
Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol.[10] Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.[10][11]

B. Steroidogenesis
Steroidogenesis is the biological process by which steroids are generated from cholesterol and transformed into other steroids. The pathways of steroidogenesis differ between different species, but the pathways of human steroidogenesis are shown in the figure.
Products of steroidogenesis include:
C. Elimination
Steroids are oxidized mainly by cytochrome P450 oxidase enzymes, such as CYP3A4. These reactions introduce oxygen into the steroid ring and allows the structure to be broken up by other enzymes, to form bile acids as final products.[12] These bile acids can then be eliminated through secretion from the liver in the bile.[13] The expression of this oxidase gene can be upregulated by the steroid sensor PXR when there is a high blood concentration of steroids.[14]
See also
External links
- Nomenclature of Steroids Home Page at Queen Mary University of London.
- Steroidogenesis
References
- ^ a b G. P. Moss (1989). "Nomenclature of Steroids (Recommendations 1989)". Pure & Appl. Chem. 61 (10): 1783–1822. doi:10.1351/pac198961101783. PDF "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). The nomenclature of steroids. Recommendations 1989". Eur. J. Biochem. 186 (3): 429–58. 1989. doi:10.1111/j.1432-1033.1989.tb15228.x. PMID 2606099.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lanosterol biosynthesis
- ^ PubChem; CAS Number: 203-64-5
- ^ britannica.com > Facts about gonane: steroids, as discussed in steroid (chemical compound): Steroid numbering system and nomenclature Retrieved on Feb 13, 2010
- ^ Rossier MF (2006). "T channels and steroid biosynthesis: in search of a link with mitochondria". Cell Calcium. 40 (2): 155–64. doi:10.1016/j.ceca.2006.04.020. PMID 16759697.
- ^ Grochowski L, Xu H, White R (2006). "Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate". J Bacteriol. 188 (9): 3192–8. doi:10.1128/JB.188.9.3192-3198.2006. PMC 1447442. PMID 16621811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lichtenthaler H (1999). "The 1-Dideoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annu Rev Plant Physiol Plant Mol Biol. 50: 47–65. doi:10.1146/annurev.arplant.50.1.47. PMID 15012203.
- ^ a b Kuzuyama T, Seto H (2003). "Diversity of the biosynthesis of the isoprene units". Nat Prod Rep. 20 (2): 171–83. doi:10.1039/b109860h. PMID 12735695.
- ^ Dubey V, Bhalla R, Luthra R (2003). "An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants" (PDF). J Biosci. 28 (5): 637–46. doi:10.1007/BF02703339. PMID 14517367.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Schroepfer G (1981). "Sterol biosynthesis". Annu Rev Biochem. 50: 585–621. doi:10.1146/annurev.bi.50.070181.003101. PMID 7023367.
- ^ Lees N, Skaggs B, Kirsch D, Bard M (1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—a review". Lipids. 30 (3): 221–6. doi:10.1007/BF02537824. PMID 7791529.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pikuleva IA (2006). "Cytochrome P450s and cholesterol homeostasis". Pharmacol. Ther. 112 (3): 761–73. doi:10.1016/j.pharmthera.2006.05.014. PMID 16872679.
- ^ Zollner G, Marschall HU, Wagner M, Trauner M (2006). "Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations". Mol. Pharm. 3 (3): 231–51. doi:10.1021/mp060010s. PMID 16749856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kliewer S, Goodwin B, Willson T (2002). "The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism". Endocr. Rev. 23 (5): 687–702. doi:10.1210/er.2001-0038. PMID 12372848.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
