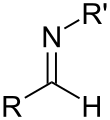
Imine

In organic chemistry, an imine (/ɪˈmiːn/ or /ˈɪmɪn/) is a functional group or organic compound containing a carbon–nitrogen double bond (C=N). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds.[1][2] Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.[3]
Distinction is sometimes made between aldimines and ketimines, derived from aldehydes and ketones, respectively.
Structure
[edit]In imines the five core atoms (C2C=NX, ketimine; and C(H)C=NX, aldimine; X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29–1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C−N distances in amines and nitriles are 1.47 and 1.16 Å respectively.[4] Rotation about the C=N bond is slow. Using NMR spectroscopy, both E and Z isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored.[5]
Nomenclature and classification
[edit]The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.[6]
Usually imines refer to compounds with the general formula R2C=NR, as discussed below.[7] In the older literature, imine refers to the aza-analogue of an epoxide. Thus, ethylenimine is the three-membered ring species aziridine C2H4NH.[8] The relationship of imines to amines having double and single bonds can be correlated with imides and amides, as in succinimide vs acetamide.
Imines are related to ketones and aldehydes by replacement of the oxygen with an NR group. When R = H, the compound is a primary imine, when R is hydrocarbyl, the compound is a secondary imine. If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base.[9] When R3 is OH, the imine is called an oxime, and when R3 is NH2 the imine is called a hydrazone.
A primary imine in which C is attached to both a hydrocarbyl and a H (derived from an aldehyde) is called a primary aldimine; a secondary imine with such groups is called a secondary aldimine.[10] A primary imine in which C is attached to two hydrocarbyls (derived from a ketone) is called a primary ketimine; a secondary imine with such groups is called a secondary ketimine.[11]
-
Primary aldimine, E-isomer
-
Secondary aldimine, E-isomer
-
Primary ketimine
-
Secondary ketimine
N-Sulfinyl imines are a special class of imines having a sulfinyl group attached to the nitrogen atom.
Synthesis of imines
[edit]Carbonyl-amine condensation
[edit]Imines are typically prepared by the condensation of primary amines and aldehydes.[12][13] Ketones undergo similar reactions, but less commonly than aldehydes. In terms of mechanism, such reactions proceed via the nucleophilic addition giving a hemiaminal -C(OH)(NHR)- intermediate, followed by an elimination of water to yield the imine (see alkylimino-de-oxo-bisubstitution for a detailed mechanism). The equilibrium in this reaction usually favors the carbonyl compound and amine, so that azeotropic distillation or use of a dehydrating agent, such as molecular sieves or magnesium sulfate, is required to favor of imine formation. In recent years, several reagents such as Tris(2,2,2-trifluoroethyl)borate [B(OCH2CF3)3],[14] pyrrolidine[15] or titanium ethoxide [Ti(OEt)4][16] have been shown to catalyse imine formation.
Rarer than primary amines is the use of ammonia to give a primary imine.[17] In the case of hexafluoroacetone, the hemiaminal intermediate can be isolated.[18]
From nitriles
[edit]Primary ketimines can be synthesized via a Grignard reaction with a nitrile. This method is known as Moureu-Mignonac ketimine synthesis.[19] [20][21] For example, benzophenone imine can also be synthesized by addition of phenylmagnesium bromide to benzonitrile followed by careful hydrolysis (lest the imine be hydrolyzed):[22]
- C6H5CN + C6H5MgBr → (C6H5)2C=NMgBr
- (C6H5)2C=NMgBr + H2O → (C6H5)2C=NH + MgBr(OH)
Specialized methods
[edit]Several other methods exist for the synthesis of imines.
- Reaction of organic azides with metal carbenoids (produced from diazocarbonyl compounds).[23]
- The reaction of iminophosphoranes and organic azides in an Aza-Wittig-reaction.
- Condensation of carbon acids with nitroso compounds.
- The rearrangement of trityl N-haloamines in the Stieglitz rearrangement.
- By reaction of alkenes with hydrazoic acid in the Schmidt reaction.
- By reaction of a nitrile, hydrochloric acid, and an arene in the Hoesch reaction.
- Multicomponent synthesis of 3-thiazolines in the Asinger reaction.
- Thermal decomposition of oximes.[24]
Reactions
[edit]
Hydrolysis
[edit]The chief reaction of imines, often undesirable, is their hydrolysis back to the amine and the carbonyl precursor.
- R2C=NR' + H2O ⇌ R2C=O + R'NH2
Precursors to heterocycles
[edit]Imines are widely used as intermediates in the synthesis of heterocycles.
- Aromatic imines react with an enol ether to a quinoline in the Povarov reaction.
- Imines react, thermally, with ketenes in [2+2] cycloadditions to form β-lactams in the Staudinger synthesis.[25] Several variants have been described.[26][27]
- Imine react with dienes in the Imine Diels-Alder reaction to a tetrahydropyridine.
- tosylimines react with α,β-unsaturated carbonyl compound to give allylic amines in the Aza-Baylis–Hillman reaction.
Acid-base reactions
[edit]Somewhat like the parent amines, imines are mildly basic and reversibly protonate to give iminium salts:
- R2C=NR' + H+ [R2C=NHR']+
Alternatively, primary imines are sufficiently acidic to allow N-alkylation, as illustrated with benzophenone imine:[28]
- (C6H5)2C=NH + CH3Li → (C6H5)2C=NLi + CH4
- (C6H5)2C=NLi + CH3I → (C6H5)2C=NCH3 + LiI
Lewis acid-base reactions
[edit]Imines are common ligands in coordination chemistry. Particularly popular examples are found with Schiff base ligands derived from salicylaldehyde, the salen ligands. Metal-catalyzed reactions of imines proceed through such complexes. In classical coordination complexes, imines bind metals through nitrogen. For low-valent metals, η2-imine ligands are observed.
Nucleophilic additions
[edit]Very analogous to ketones and aldehydes, primary imines are susceptible to attack by carbanion equivalents. The method allow for the synthesis of secondary amines:[29][30]
- R2C=NR' + R"Li → R2R"CN(Li)R'
- R2R"CN(Li)R' + H2O → R2R"CNHR' + LiOH
This can be expanded to include enolisable carbons in the Mannich reaction, which is a straightforward and commonly used approach for producing β-amino-carbonyl compounds.[31]
Imine reductions
[edit]Imines are reduced via reductive amination. An imine can be reduced to an amine via hydrogenation for example in a synthesis of m-tolylbenzylamine:[32]
Other reducing agents are lithium aluminium hydride and sodium borohydride.[33]
The asymmetric reduction of imines has been achieved by hydrosilylation using a rhodium-DIOP catalyst.[34] Many systems have since been investigated.[35][36]
Owing to their enhanced electrophilicity, iminium derivatives are particularly susceptible to reduction to the amines. Such reductions can be achieved by transfer hydrogenation or by the stoichiometric action of sodium cyanoborohydride. Since imines derived from unsymmetrical ketones are prochiral, their reduction defines a route to chiral amines.
Polymerisation
[edit]Unhindered aldimines tend to cyclize, as illustrated by the condensation of methylamine and formaldehyde, which gives the hexahydro-1,3,5-triazine.
Imine polymers (polyimines) can be synthesised from multivalent aldehydes and amines.[37] The polymerisation reaction proceeds directly when the aldehyde and amine monomers are mixed together at room temperature. In most cases, (small) amounts of solvent may still be required. Polyimines are particularly interesting materials because of their application as vitrimers. Owing to the dynamic covalent nature of the imine bonds, polyimines can be recycled relatively easily. Furthermore, polyimines are known for their self-healing behaviour.[38][39]
Miscellaneous reactions
[edit]Akin to pinacol couplings, imines are susceptible to reductive coupling leading to 1,2-diamines. [40]
Imine are oxidized with meta-chloroperoxybenzoic acid (mCPBA) to give an oxaziridines.
Imines are intermediates in the alkylation of amines with formic acid in the Eschweiler-Clarke reaction.
A rearrangement in carbohydrate chemistry involving an imine is the Amadori rearrangement.
A methylene transfer reaction of an imine by an unstabilised sulphonium ylide can give an aziridine system. Imine react with dialkylphosphite in the Pudovik reaction and Kabachnik–Fields reaction
Biological role
[edit]Imines are common in nature.[41][42] The pyridoxal phosphate-dependent enzymes (PLP enzymes) catalyze myriad reactions involving aldimines (or Schiff bases).[43] Cyclic imines are also substrates for many imine reductase enzymes.[44]

See also
[edit]- Enamine
- Schiff base
- Carboximidate
- Oxazolidine
- Other functional groups with a C=N double bond: oximes, hydrazones
- Other functional groups with a C N triple bond: nitriles, isonitriles
References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "imines". doi:10.1351/goldbook.I02957
- ^ March, Jerry (1985). Advanced Organic Chemistry Reactions, Mechanisms and Structure (3rd ed.). New York: Wiley, inc. ISBN 0-471-85472-7. OCLC 642506595.
- ^ Saul Patai, ed. (1970). Carbon–Nitrogen Double Bonds. PATai's Chemistry of Functional Groups. John Wiley & Sons. doi:10.1002/9780470771204. ISBN 9780471669425. OCLC 639112179.
- ^ C. Sandorfy (1970). "General and theoretical aspects". In Saul Patai (ed.). Carbon–Nitrogen Double Bonds. PATai's Chemistry of Functional Groups. John Wiley & Sons. pp. 1–60. doi:10.1002/9780470771204.ch1. ISBN 9780470771204.
- ^ Bjørgo, Johannes; Boyd, Derek R.; Watson, Christopher G.; Jennings, W. Brian; Jerina, Donald M. (1974). "E–Z-isomerism in Aldimines". J. Chem. Soc., Perkin Trans. 2 (9): 1081–1084. doi:10.1039/P29740001081.
- ^ Ladenburg, A. (1883). "Ueber die Imine" [About imines]. Berichte der Deutschen Chemischen Gesellschaft (in German). 16: 1149–1152. doi:10.1002/cber.188301601259.
From p. 1150: Denn offenbar gehört auch das Piperidin in die Klasse der von mir gesuchten Verbindungen, für welche der Name Imine durch die bestehende Nomenklatur angezeigt ist.
[For obviously piperidine also belongs in the class of compounds that are sought by me, for which the name "imines" is indicated by the prevailing nomenclature.] - ^ "Amines and Imines". Nomenclature of Organic Compounds. Advances in Chemistry. Vol. 126. American Chemical Society. 1974. pp. 180–188. doi:10.1021/ba-1974-0126.ch023. ISBN 9780841201910. OCLC 922539.
- ^ "Ethylenimine". Organic Syntheses. 30: 38. 1950. doi:10.15227/orgsyn.030.0038.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff base". doi:10.1351/goldbook.S05498
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aldimines". doi:10.1351/goldbook.A00209.html
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "ketimines". doi:10.1351/goldbook.K03381.html
- ^ G. Wittig, A. Hesse (1970). "Directed Aldol Condensations:b-Phenylcinnamaldehyde". Organic Syntheses. 50: 66. doi:10.15227/orgsyn.050.0066.
- ^ Bigelow, Lucius A.; Eatough, Harry (1928). "Benzalaniline". Organic Syntheses. 8: 22. doi:10.15227/orgsyn.008.0022.
- ^ Reeves, Jonathan T.; Visco, Michael D.; Marsini, Maurice A.; Grinberg, Nelu; Busacca, Carl A.; Mattson, Anita E.; Senanayake, Chris H. (2015-05-15). "A General Method for Imine Formation Using B(OCH2CF3)3". Organic Letters. 17 (10): 2442–2445. doi:10.1021/acs.orglett.5b00949. ISSN 1523-7060. PMID 25906082.
- ^ Morales, Sara; Guijarro, Fernando G.; García Ruano, José Luis; Cid, M. Belén (2014-01-22). "A General Aminocatalytic Method for the Synthesis of Aldimines". Journal of the American Chemical Society. 136 (3): 1082–1089. doi:10.1021/ja4111418. ISSN 0002-7863. PMID 24359453.
- ^ Collados, Juan F.; Toledano, Estefanía; Guijarro, David; Yus, Miguel (2012-07-06). "Microwave-Assisted Solvent-Free Synthesis of Enantiomerically Pure N-(tert-Butylsulfinyl)imines". The Journal of Organic Chemistry. 77 (13): 5744–5750. doi:10.1021/jo300919x. ISSN 0022-3263. PMID 22694241.
- ^ Verardo, G.; Giumanini, A. G.; Strazzolini, P.; Poiana, M. (1988). "Ketimines From Ketones and Ammonia". Synthetic Communications. 18 (13): 1501–1511. doi:10.1080/00397918808081307.
- ^ a b Middleton, W. J.; Carlson, H. D. (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081.
- ^ "Moureau-Mignonac Ketimine Synthesis". Comprehensive Organic Name Reactions and Reagents. Hoboken, NJ, USA: John Wiley & Sons, Inc. 2010-09-15. pp. 1988–1990. doi:10.1002/9780470638859.conrr446. ISBN 9780470638859.
- ^ Koos, Miroslav; Mosher, Harry S. (1993). "α-Amino-α-trifluoromethyl-phenylacetonitrile: A potential reagent for NMR determination of enantiomeric purity of acids". Tetrahedron. 49 (8): 1541–1546. doi:10.1016/S0040-4020(01)80341-0.
- ^ Moureu, Charles; Mignonac, Georges (1920). "Les Cetimines". Annales de Chimie. 9 (13): 322–359. Retrieved 18 June 2014.
- ^ Pickard, P. L.; Tolbert, T. L. (December 1961). "An Improved Method of Ketimine Synthesis". The Journal of Organic Chemistry. 26 (12): 4886–4888. doi:10.1021/jo01070a025. ISSN 0022-3263.
- ^ Mandler, Michael; Truong, Phong; Zavalij, Peter; Doyle, Michael (Jan 14, 2014). "Catalytic Conversion of Diazocarbonyl Compounds to Imines: Applications to the Synthesis of Tetrahydropyrimidines and β-Lactams". Organic Letters. 16 (3): 740–743. doi:10.1021/ol403427s. PMID 24423056.
- ^ Arthur Lachman (1930). "Diphenylmethane Imine Hydrochloride". Organic Syntheses. 10: 28. doi:10.15227/orgsyn.010.0028.
- ^ Hubschwerlen, Christian; Specklin, Jean-Luc (1995). "(3S,4S)-3-Amino-1-(3,4-Dimethoxybenzyl)-4-[(R)-2,2-Dimethyl-1,3-Dioxolan-4-Yl]-2-Azetidinone". Organic Syntheses. 72: 14. doi:10.15227/orgsyn.072.0014.
- ^ Hegedus, Lous S.; McGuire, Michael A.; Schultze, Lisa M. (1987). "1,3-Dimethyl-3-Methoxy-4-Phenylazetidinone". Organic Syntheses. 65: 140. doi:10.15227/orgsyn.065.0140.
- ^ Ian P. Andrews and Ohyun Kwon (2011). "PHOSPHINE-CATALYZED [3 + 2] ANNULATION: SYNTHESIS OF ETHYL 5-(tert-BUTYL)-2-PHENYL-1-TOSYL-3-PYRROLINE-3-CARBOXYLATE". Organic Syntheses. 88: 138. doi:10.15227/orgsyn.088.0138.
- ^ Nottingham, Chris; Lloyd-Jones, Guy C. (2018). "Trimethylsilyldiazo[13C]methane: A Versatile 13C-Labelling Reagent". Organic Syntheses. 95: 374–402. doi:10.15227/orgsyn.095.0374. hdl:20.500.11820/c801073c-6b4b-4a85-be68-2c4313b6e53d.
- ^ Hu, Anjing; Zhang, Zhan-Ming; Xiao, Yuanjing; Zhang, Junliang (2020). "Stereoselective Synthesis of Chiral Sulfinamide Monophosphine Ligands (Ming-Phos)(S, Rs)-M". Organic Syntheses. 97: 262–273. doi:10.15227/orgsyn.097.0262. S2CID 235020219.
- ^ Vincent Rodeschini, Nigel S. Simpkins, and Fengzhi Zhang (2007). "Chiral Lithium Amide Base Desymmetrization of a Ring Fused Imide: Formation of (3aS,7aS)-2[2-(3,4-Dimethoxyphenyl)-ethyl]-1,3-dioxo-octahydro-isoindole-3a-Carboxylic Acid Methyl Ester". Organic Syntheses. 84: 306. doi:10.15227/orgsyn.084.0306.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Arend, Michael; Westermann, Bernhard; Risch, Nikolaus (4 May 1998). "Modern Variants of the Mannich Reaction". Angewandte Chemie International Edition. 37 (8): 1044–1070. doi:10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E.
- ^ C. F. H. Allen and James VanAllan (1955). "m-Tolylbenzylamine". Organic Syntheses: 827; Collected Volumes, vol. 3.
- ^ For example: Ieva R. Politzer and A. I. Meyers (1988). "Aldehydes from 2-Benzyl-4,4,6-trimethyl-5,6-dihydro-1,3(4H)-oxazine: 1-Phenylcyclopentanecarboxaldehyde". Organic Syntheses; Collected Volumes, vol. 6, p. 905.
- ^ Langlois, N (1973). "Synthese asymetrique d'amines par hydrosilylation d'imines catalysee par un complexe chiral du rhodium". Tetrahedron Lett. 14 (49): 4865–4868. doi:10.1016/S0040-4039(01)87358-5.
- ^ Kobayashi, Shū; Ishitani, Haruro (1999). "Catalytic Enantioselective Addition to Imines". Chem. Rev. 99 (5): 1069–94. doi:10.1021/cr980414z. PMID 11749440.
- ^ J. Martens: Reduction of Imino Groups (C=N) in (G. Helmchen, R. W. Hoffmann, J. Mulzer, E. Schaumann) Houben-Weyl Stereoselective Synthesis, Workbench Edition E21 Volume 7, S. 4199-4238, Thieme Verlag Stuttgart, 1996, ISBN 3-13-106124-3.
- ^ Schoustra, Sybren K.; Groeneveld, Timo; Smulders, Maarten M. J. (2021). "The effect of polarity on the molecular exchange dynamics in imine-based covalent adaptable networks". Polymer Chemistry. 12 (11): 1635–1642. doi:10.1039/D0PY01555E.
- ^ Schoustra, Sybren K.; Dijksman, Joshua A.; Zuilhof, Han; Smulders, Maarten M. J. (2021). "Molecular control over vitrimer-like mechanics – tuneable dynamic motifs based on the Hammett equation in polyimine materials". Chemical Science. 12 (1): 293–302. doi:10.1039/d0sc05458e. ISSN 2041-6520. PMC 8178953. PMID 34163597.
- ^ Zhu, Jiaqi (2020). "A self-healing transparent polydimethylsiloxane elastomer based on imine bonds". European Polymer Journal. 123: 109382. Bibcode:2020EurPJ.12309382W. doi:10.1016/j.eurpolymj.2019.109382. S2CID 214199868.
- ^ Alexakis, Alex; Aujard, Isabelle; Kanger, Tonis; Mangeney, Pierre (1999). "(R,R)- and (S,S)-N,N'-Dimethyl-1,2-Diphenylethylene-1,2-Diamine". Organic Syntheses. 76: 23. doi:10.15227/orgsyn.076.0023.
- ^ "Researchers look to nature to unearth the secrets of cyclic imine cleavage". EurekAlert!. Retrieved 2021-07-22.
- ^ Borchert, Andrew J.; Ernst, Dustin C.; Downs, Diana M. (2019). "Reactive Enamines and Imines in vivo: Lessons from the RidA Paradigm". Trends in Biochemical Sciences. 44 (10): 849–860. doi:10.1016/j.tibs.2019.04.011. ISSN 0968-0004. PMC 6760865. PMID 31103411.
- ^ Eliot, Andrew C.; Kirsch, Jack F. (2004). "Pyridoxal Phosphate Enzymes: Mechanistic, Structural, and Evolutionary Considerations". Annual Review of Biochemistry. 73: 383–415. doi:10.1146/annurev.biochem.73.011303.074021. PMID 15189147.
- ^ Mangas-Sanchez, Juan; France, Scott P; Montgomery, Sarah L; Aleku, Godwin A; Man, Henry; Sharma, Mahima; Ramsden, Jeremy I; Grogan, Gideon; Turner, Nicholas J (2017). "Imine reductases (IREDs)". Current Opinion in Chemical Biology. 37: 19–25. doi:10.1016/j.cbpa.2016.11.022. PMID 28038349.