Morphogenesis: Difference between revisions
| Line 19: | Line 19: | ||
===Extracellular Matrix=== |
===Extracellular Matrix=== |
||
The [[extracellular matrix]] (ECM) is involved with separating tissues, providing structural support or providing a structure for cells to migrate on. Collagen, laminin, and fibronectin are the major molecules and are secreted and assembled into sheets, fibers and gels. Multisubunit transmembrane receptors called integrins are used to bind to the ECM. [[Integrins]] bind extracellularly to fibronectin, laminin or other ECM components and intracellularly to microfilament binding proteins a-actinin and talin to link the cytoskeleton with the outside. Integrins also serve as receptors to trigger signal transduction cascades when binding to the ECM. |
The [[extracellular matrix]] (ECM) is involved with separating tissues, providing structural support or providing a structure for cells to migrate on. Collagen, laminin, and fibronectin are the major molecules and are secreted and assembled into sheets, fibers and gels. Multisubunit transmembrane receptors called integrins are used to bind to the ECM. [[Integrins]] bind extracellularly to fibronectin, laminin or other ECM components and intracellularly to microfilament binding proteins a-actinin and talin to link the cytoskeleton with the outside. Integrins also serve as receptors to trigger signal transduction cascades when binding to the ECM. |
||
YO DALILAH LOVES KEVIN'S PENIS |
|||
==Anterior-posterior axis patterning in ''Drosophila''== |
==Anterior-posterior axis patterning in ''Drosophila''== |
||
Revision as of 12:25, 22 June 2007
Morphogenesis (from the Greek morphê shape and genesis creation) is one of three fundamental aspects of developmental biology along with the control of cell growth and cellular differentiation. Morphogenesis is concerned with the shapes of tissues, organs and entire organisms and the positions of the various specialized cell types. Cell growth and differentiation can take place in cell culture or inside of tumor cell masses without the normal morphogenesis that is seen in an intact organism. The study of morphogenesis involves an attempt to understand the processes that control the organized spatial distribution of cells that arises during the embryonic development of an organism and which give rise to the characteristic forms of tissues, organs and overall body anatomy. In the human embryo, the change from a cluster of nearly identical cells at the blastula stage to a post-gastrulation embryo with structured tissues and organs is controlled by the genetic "program" and can be modified by environmental factors.
History
Some of the earliest ideas on how physical and mathematical processes and constraints affect biological growth were written by D'Arcy Wentworth Thompson and Alan Turing. These works postulated the presence of chemical signals and physico-chemical processes such as diffusion, activation and deactivation in cellular and organismic growth. The fuller understanding of the mechanisms involved in actual organisms required the discovery of DNA and the development of molecular biology and biochemistry.
Molecular basis
Several types of molecules are particularly important during morphogenesis. Morphogens are soluble molecules that can diffuse and carry signals that control cell differentiation decisions in a concentration-dependent fashion. Morphogens typically act through binding to specific protein receptors. An important class of molecules involved in morphogenesis are transcription factor proteins that determine the fate of cells by interacting with DNA. These can be coded for by master regulatory genes and either activate or deactivate the transcription of other genes and, in turn, these secondary gene products can regulate the expression of still other genes in a regulatory cascade. Another class of molecules involved in morphogenesis are molecules that control cell adhesion. For example, during gastrulation clumps of stem cells switch off their cell-to-cell adhesion, become migratory, and take up new positions within an embryo where they again activate specific cell adhesion proteins and form new tissues and organs. Several examples that illustrate the roles of morphogens, transcription factors and cell adhesion molecules in morphogenesis are discussed below.
Cellular basis
Morphogenesis arises because of changes in the cellular structure. Certain cell types sort out. The ability of cells to do this comes from differential changes in cellular structure. There are two types of cells epithelial cells and mesenchymal cells. By changing from one cell type to another, cells are able to move around and associate with other like cells.
Adhesion
Cells sort out in different layers due to the differential adhesion model or thermodynamic model. This model states that cells sort based upon differences in adhesion between the cells. Cells that move to the center of a mixed aggregate of cells have the strongest adhesion.
The molecules responsible for adhesion are called cell adhesion molecules (CAMs). Several types of cell adhesion molecules are known and one major class of these molecules are cadherins. Cadherins are a calcium dependent transmembrane protein that connects to the actin cytoskeleton through binding to trimeric proteins made up of a, b, and g catenins. Cadherins bind to other cadherins in a like-like manner (E-cadherins bind to other E-cadherins) or homophilic interactions.
Extracellular Matrix
The extracellular matrix (ECM) is involved with separating tissues, providing structural support or providing a structure for cells to migrate on. Collagen, laminin, and fibronectin are the major molecules and are secreted and assembled into sheets, fibers and gels. Multisubunit transmembrane receptors called integrins are used to bind to the ECM. Integrins bind extracellularly to fibronectin, laminin or other ECM components and intracellularly to microfilament binding proteins a-actinin and talin to link the cytoskeleton with the outside. Integrins also serve as receptors to trigger signal transduction cascades when binding to the ECM. YO DALILAH LOVES KEVIN'S PENIS
Anterior-posterior axis patterning in Drosophila
One of the best understood morphogenetic systems is the patterning along the future head to tail (antero-posterior) axis of the fruit fly Drosophila melanogaster. The development of Drosophila is particularly well studied, and it is representative of a major class of animals, the insects or insecta. Other multicellular organisms sometimes use similar mechanisms for axis formation, although the relative importance of signal transfer between the earliest cells of many developing organisms is greater than in the example described here.
Maternal effect genes


The building-blocks of anterior-posterior axis patterning in Drosophila are laid out during egg formation (oogenesis), well before the egg is fertilized and deposited. The developing egg (oocyte) is polarized by differentially localized mRNA molecules.
The genes that code for these mRNAs, called maternal effect genes, encode for proteins that get translated upon fertilization to establish concentration gradients that span the egg. Bicoid and hunchback are the maternal effect genes that are most important for patterning of anterior parts (head and thorax) of the Drosophila embryo. Nanos and Caudal are maternal effect genes that are important in the formation of more posterior abdominal segments of the Drosophila embryo.
Cytoskeletal elements such as microtubules are polarized within the oocyte and can be used to allow the localization of mRNA molecules to specific parts of the cell. Maternally synthesized bicoid mRNAs attach to microtubules and are concentrated at the anterior ends of forming Drosophila eggs. Nanos mRNAs also attach to the egg cytoskeleton but they concentrate at the posterior ends of the eggs. Hunchback and caudal mRNAs lack special location control systems and are fairly evenly spread throughout the interior of egg cells.
When the mRNAs from the maternal effect genes are translated into proteins a Bicoid protein gradient forms at the anterior end of the egg. Nanos protein forms a gradient at the posterior end. The Bicoid protein blocks translation of caudal mRNA so Caudal protein is made only in the posterior part of the cell. Nanos protein binds to the hunchback mRNA and blocks its translation in the posterior end of Drosophila embryos.
The Bicoid, Hunchback, and Caudal proteins are transcription factors. Bicoid has a DNA-binding homeodomain that binds both DNA and the nanos mRNA. Bicoid binds a specific RNA sequence in the 3' untranslated region of caudal mRNA and blocks translation.
Hunchback protein levels in the early embryo are significantly augmented by new hunchback gene transcription and translation of the resulting zygotically produced mRNA. During early Drosophila embryogenesis there are nuclear divisions without cell division. The many nuclei that are produced distribute themselves around the periphery of the cell cytoplasm. Gene expression in these nuclei is regulated by the Bicoid, Hunchback, and Caudal proteins. For example, Bicoid acts as a transcriptional activator of hunchback gene transcription.


Gap genes
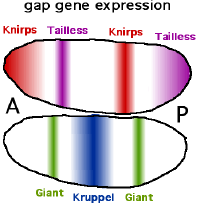
The other important function of the gradients of Bicoid, Hunchback, and Caudal proteins is in the transcriptional regulation of other zygotically expressed proteins. Many of these are the protein products derived from members of the "gap" family of developmental control genes. Hunchback, krüppel, giant, tailless and knirps are all gap genes. Their expression patterns in the early embryo are determined by the maternal effect gene products and shown in the diagrams on the right side of this page. The gap genes are part of a larger family called the segmentation genes. These genes establish the segmented body plan of the embryo along the anterior-posterior axis. The segmentation genes specify 14 "parasegments" that are closely related to the final anatomical segments. The gap genes are the first layer of a hierarchical cascade of the segmentation control genes.
Proteins such as Bicoid can be described as morphogens that act within the syncytial blastoderm of the early Drosophila embryo. These intracellular morphogens enter the nuclei and act as transcription factors to control expression of the gap genes.
In the blastoderm stage of Drosophila morphogenesis four types of nuclear specification can be distinguished:
- Anterior (head and thorax)
- Posterior (abdomen)
- Dorso-ventral
- Terminal (special structures at the unsegmented ends of the embryo)
Additional segmentation genes
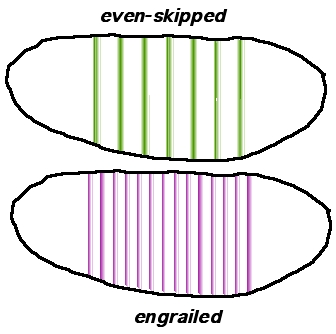
Two additional classes of segmentation genes are expressed after the gap gene products. The pair-rule genes are expressed in striped patterns of seven bands perpendicular to the anterior-posterior axis (see Figure 6, even-skipped). These patterns of expression are established within the syncytial blastoderm. After these initial patterning events, cell membranes form around the nuclei of the syncytial blastoderm converting it to a cellular blastoderm.

The expression patterns of the final class of segmentation genes, the segment polarity genes, are then fine-tuned by interactions between the cells of adjacent parasegments (see the example, engrailed, Figure 7). The Engrailed protein is a transcription factor (yellow in Figure 7) that is expressed in one row of cells at the edge of each parasegment. This expression pattern is initiated by the pair-rule genes (like even-skipped) that code for transcription factors that regulate the engrailed gene's transcription in the syncytial blastoderm.
Cells that make Engrailed can make the cell-to-cell signaling protein Hedgehog (green in Figure 7). Hedgehog is not free to move very far and activates a thin stripe of cells adjacent to the Engrailed-expressing cells. Only cells to one side of the Engrailed-expressing cells are competent to respond to Hedgehog because they express the receptor protein Patched (blue in Figure 7). Cells with activated Patch receptor make the Wingless protein (red in Figure 7). Wingless protein acts the adjacent rows of cells by activated its cell surface receptor, Frizzled.
Wingless also acts on Engrailed-expressing cells to stabilize Engrailed expression after the cellular blastoderm forms. The reciprocal signaling by Hedgehog and Wingless stabilizes the boundary between each segment. The Wingless protein is called "wingless" because of the phenotype of some wingless mutants. Wingless also functioned during metamorphosis to coordinate wing formation.
The transcription factors that are coded for by segmentation genes regulate yet another family of developmental control genes, the homeotic selector genes. These genes exist in two ordered groups on Drosophila chromosome 3. The order of the genes on the chromosome reflects the order that they are expressed along the anterior-posterior axis of the developing embryo. The Antennapedia group of homeotic selector genes includes labial, antennapedia, sex combs reduced, deformed, and proboscipedia. Labial and Deformed proteins are expressed in head segments where they activate the genes that define head features. Sex-combs-reduced and Antennapedia specify the properties of thoracic segments. The bithorax group of homeotic selector genes control the specializations of the third thoracic segment and the abdominal segments.
In 1995, the Nobel Prize for Physiology or Medicine was awarded for studies concerning the genetic control of early embryonic development to Christiane Nüsslein-Volhard, Edward B. Lewis and Eric Wieschaus. Their researches on genetic screening for embryo patterning mutants revealed the role played in early embryologic development by Homeobox genes like bicoid. An example of a homeotic mutation is the so-called antennapedia mutation. In Drosophila, antennae and legs are created by the same basic "program", they only differ in a single transcription factor. If this transcription factor is damaged, the fly grows legs on its head instead of antennae. See images of this "antennapedia" mutant and others, at FlyBase.
The term morphogenesis can also be used to describe the development of unicellular life forms that do not have an embryonic stage in their life cycle, or to refer to the evolution of a body structure within a taxonomic group. Morphogenetic responses may be induced in organisms by hormones, or by environmental chemicals ranging from substances produced by other organisms to toxic chemicals or radionuclides released as pollutants, and other plants.
