Formaldehyde: Difference between revisions
←Replaced content with '{{for|the 1993 rock album|Formaldehyde (album)}}' |
m Reverted edits by 32.165.129.208 to last revision by LorenzoB (HG) |
||
| Line 1: | Line 1: | ||
{{for|the 1993 rock album|Formaldehyde (album)}} |
{{for|the 1993 rock album|Formaldehyde (album)}} |
||
{{chembox |
|||
| Name = Formaldehyde (methanal) |
|||
| ImageFile = Formaldehyde-2D.svg |
|||
| ImageSize = 120px |
|||
| ImageFileL1 = Formaldehyde-3D-balls-A.png |
|||
| ImageSizeL1 = 120px |
|||
| ImageFileR1 = Formaldehyde-3D-vdW.png |
|||
| ImageSizeR1 = 120px |
|||
| IUPACName= Methanal |
|||
| OtherNames = formol, methyl aldehyde, methylene oxide| Section1 = {{Chembox Identifiers |
|||
| SMILES = C=O |
|||
| CASNo = 50-00-0 |
|||
| CASNo_Ref = {{cascite}} |
|||
| ChemSpiderID = 692 |
|||
| RTECS = LP8925000 |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| Formula = CH<sub>2</sub>O |
|||
| MolarMass = 30.03 g·mol<sup>−1</sup> |
|||
| Appearance = colorless gas |
|||
| Density = 1 kg·m<sup>−3</sup>, gas |
|||
| Solubility = > 100 g/100 ml (20 °C) |
|||
| MeltingPt = -117 °C (156 [[Kelvin|K]]) |
|||
| BoilingPt = -19.3 °C (253.9 K) |
|||
}} |
|||
| Section3 = {{Chembox Structure |
|||
| MolShape = trigonal planar |
|||
| Dipole = 2.33168(1) [[Debye|D]] |
|||
}} |
|||
| Section7 = {{Chembox Hazards |
|||
| MainHazards = Toxic, Flammable, Carcinogenic |
|||
| NFPA-H = 3 |
|||
| NFPA-F = 2 |
|||
| NFPA-R = 2 |
|||
| FlashPt = -53 °C |
|||
| RPhrases = {{R23/24/25}}, {{R34}}, {{R40}}, {{R43}}| SPhrases = {{S1/2}}, {{S26}}, {{S36/37}}, {{S39}}, {{S45}}, {{S51}} |
|||
}} |
|||
| Section8 = {{Chembox Related |
|||
| Function = [[aldehydes]] |
|||
| OtherFunctn = [[acetaldehyde]] |
|||
[[benzaldehyde]] |
|||
| OtherCpds = [[ketone]]s |
|||
[[carboxylic acid]]s}} |
|||
}} |
|||
'''Formaldehyde''' (IUPAC name '''methanal''') is a [[chemical compound]] with the [[chemical formula|formula]] H<sub>2</sub>CO. It is the simplest [[aldehyde]]. Formaldehyde exists in several forms aside from H<sub>2</sub>CO: the cyclic trimer [[trioxane]] and the polymer [[Polyoxymethylene|paraformaldehyde]]. It exists in water as the hydrate H<sub>2</sub>C(OH)<sub>2</sub>. Aqueous solutions of formaldehyde are referred to as '''formalin'''. "100%" formalin consists of a saturated solution of formaldehyde (roughly 40% by mass) in water, with a small amount of [[stabilizer (chemistry)|stabilizer]], usually [[methanol]] to limit [[oxidation]] and [[polymerization]]. It is produced on a substantial scale of 6M tons/y. In view of its widespread use, toxicity, and volatility, exposure to formaldehyde is significant consideration for human health.<ref name=Ullmann>G. Reuss, W. Disteldorf, A. O. Gamer, A. Hilt, “Formaldehyde” in Ullmann’s Encyclopedia of Industrial Chemistry Wiley-VCH, 2005, Weinheim.</ref> |
|||
==Occurrence== |
|||
Formaldehyde is an intermediate in the oxidation (or [[combustion]]) of [[methane]] as well as other carbon compounds, e.g. [[forest fire]]s, in [[automobile]] exhaust, and in [[tobacco smoke]]. When produced in the [[Earth's atmosphere|atmosphere]] by the action of sunlight and [[oxygen]] on atmospheric [[methane]] and other [[hydrocarbon]]s, it becomes part of [[smog]]. |
|||
Formaldehyde, as well as its [[oligomer]]s and hydrates are rarely encountered in living organisms. [[Methanogenesis]] proceeds via the equivalent of formaldehyde, but this one-carbon species is masked as a [[methylene]] group in [[methanopterin]]. Formaldehyde is the primary cause of [[methanol]]'s toxicity, since methanol is metabolised into toxic formaldehyde by [[alcohol dehydrogenase]]. Formaldehyde is converted to [[formic acid]] in the body. Formaldehyde has been detected in outer space (see below). |
|||
==Synthesis and industrial production== |
|||
Formaldehyde was first [[Chemical synthesis|reported]] by the [[Russia]]n chemist [[Aleksandr Butlerov]] (1828-1886), but was conclusively identified by [[August Wilhelm von Hofmann]].<ref>J Read, Text-Book of Organic Chemistry, G Bell & Sons, London, 1935</ref> |
|||
Formaldehyde is produced industrially by the catalytic oxidation of [[methanol]]. The most common catalysts are [[silver]] metal or a mixture of an [[iron oxide|iron]] and [[molybdenum trioxide|molybdenum]] or [[vanadium pentoxide|vanadium]] [[oxide]]s. In the more commonly used FORMOX process methanol and oxygen react at ca 250-400 °C in presence of iron oxide in combination with molybdenum and/or vanadium to produce formaldehyde according to the [[chemical equation]]:<ref name=Ullmann>G. Reuss, W. Disteldorf, A. O. Gamer, A. Hilt, “Formaldehyde” in Ullmann’s Encyclopedia of Industrial Chemistry Wiley-VCH, 2005, Weinheim.</ref> |
|||
: 2 [[methanol|CH<sub>3</sub>OH]] + [[oxygen|O<sub>2</sub>]] → 2 H<sub>2</sub>CO + 2 [[water (molecule)|H<sub>2</sub>O]] |
|||
The silver-based catalyst is usually operated at a higher temperature, about 650 °C. Two chemical reactions on it simultaneously produce formaldehyde: that shown above and the [[dehydrogenation]] reaction: |
|||
: CH<sub>3</sub>OH → H<sub>2</sub>CO + [[hydrogen|H<sub>2</sub>]] |
|||
Formalin can be produced on a smaller scale using a whole range of other methods including conversion from ethanol instead of the normally-fed methanol feedstock. {{Fact|date=January 2009}} Such methods are of less commercial importance. |
|||
In principle formaldehyde could be generated by oxidation of [[methane]], but this route is not industrially viable because the formaldehyde is more easily oxidized than methane.<ref name=Ullmann/> |
|||
== Organic chemistry == |
|||
Formaldehyde is a building block in the synthesis of many other compounds of specialized and industrial significance. It exhibits most of the chemical properties of other aldehydes but is more reactive. For example it is more readily [[oxidation|oxidized]] by atmospheric oxygen to [[formic acid]] (formic acid is found in ppm levels in commercial formaldehyde). Formaldehyde is a good [[electrophile]], participating in [[electrophilic aromatic substitution]] reactions with [[aromatic compound]]s, and can undergo [[electrophilic addition]] reactions with [[alkene]]s and aromatics. Formaldehyde undergoes a [[Cannizzaro reaction]] in the presence of [[base (chemistry)|basic]] [[catalyst]]s to produce [[formic acid]] and methanol. |
|||
===Examples of organic synthetic applications=== |
|||
Condensation with acetaldehyde affords [[pentaerythritol]], a chemical necessary in synthesizing [[PETN]], a high explosive.<ref>{{OrgSynth | title = Pentaerythritol | author= H. B. J. Schurink | collvol = 1 | year= 1941 | volume = 1 | pages= 425 | prep = CV1P0425}}</ref> Condensation with phenols gives [[phenol-formaldehyde resin]]s. With 4-substituted phenols one obtains [[calixarenes]].<ref>{{OrgSynth | author = Gutsche, C. D.; Iqbal, M. | title = <nowiki>p-tert-Butylcalix[4]arene</nowiki> | collvol = 8 | collvolpages = 75 | year = 1993 | prep = CV8P0075}}</ref> |
|||
When combined with hydrogen sulfide it forms [[trithiane]].<ref>{{OrgSynth | author = Bost, R. W.; Constable, E. W. | title = sym-Trithiane | collvol = 2 | collvolpages = 610 | year = 1943 | prep = CV2P0610}}</ref> |
|||
: 3 CH<sub>2</sub>O + 3 H<sub>2</sub>S → (CH<sub>2</sub>S)<sub>3</sub> + 3 H<sub>2</sub>O |
|||
==Industrial applications== |
|||
Formaldehyde is a common building block for the synthesis of more complex compounds and materials. In approximate order of decreasing consumption, products generated from formaldehyde include [[urea formaldehyde resin]], [[melamine resin]], [[phenol formaldehyde resin]], [[polyoxymethylene plastic]]s, [[1,4-butanediol]], and [[methylene diphenyl diisocyanate]].<ref name=Ullmann/> |
|||
When reacted with phenol, urea, or melamine formaldehyde produces, respectively, hard thermoset phenol formaldehyde resin, urea formaldehyde resin, and melamine resin, which are commonly used in permanent adhesives such as those used in [[plywood]] or [[carpet]]ing. It is used as the wet-strength resin added to sanitary paper products such as (listed in increasing concentrations injected into the paper machine headstock chest) facial tissue, table napkins, and roll towels. They are also foamed to make [[Thermal insulation|insulation]], or [[casting|cast]] into moulded products. Production of formaldehyde resins accounts for more than half of formaldehyde consumption. |
|||
Formaldehyde is also a precursor to polyfunctional [[alcohol]]s such as [[pentaerythritol]], which is used to make [[paint]]s and [[explosive]]s. Other formaldehyde derivatives include [[methylene diphenyl diisocyanate]], an important component in [[polyurethane]] paints and foams, and [[hexamine]], which is used in phenol-formaldehyde resins as well as the explosive [[RDX]]. |
|||
The [[textile industry]] uses formaldehyde-based resins as finishers to make fabrics crease-resistant.<ref>[http://www.nicnas.gov.au/Publications/Information_Sheets/Existing_Chemical_Information_Sheets/EC_IS_Formaldehyde_102007_PDF.pdf FORMALDEHYDE IN CLOTHING AND OTHER TEXTILES]</ref> |
|||
It is also used as an ingredient by some shampoo manufacturers.{{Fact|date=September 2008}} |
|||
===Miscellaneous applications=== |
|||
Formaldehyde is a common component of many niche uses. Formaldehyde, along with 18 [[Concentration#molarity|M]] (concentrated) [[sulfuric acid]] (the entire solution often called the [[Marquis reagent]])<ref>[http://www.dancesafe.org/documents/druginfo/testkits.php DanceSafe: testing kit info<!-- Bot generated title -->]</ref>, is used as an [[MDMA]] "testing kit" by such groups as [[Dancesafe]] as well as MDMA consumers. The solution alone cannot verify the presence of MDMA but reacts with many other chemicals that the MDMA tablet itself may be adulterated with. The reaction itself produces colors that correlate with these components. |
|||
====As a disinfectant and biocide==== |
|||
An aqueous solution of formaldehyde can be useful as a disinfectant as it kills most [[bacterium|bacteria]] and fungi (including their spores). It is also used as a [[preservative]] in [[vaccine]]s. Formaldehyde solutions are applied topically in medicine to dry the skin, such as in the treatment of [[wart]]s. Many aquarists use formaldehyde as a treatment for the parasites [[ichthyophthirius]] and [[Cryptocaryon irritans]].<ref>University of Florida - Use of Formalin to Control Fish Parasites http://edis.ifas.ufl.edu/VM061</ref> |
|||
Formaldehyde preserves or [[Fixation (histology)|fixes]] tissue or cells by irreversibly cross-linking primary [[amino group]]s in proteins with other nearby nitrogen atoms in protein or [[DNA]] through a -CH<sub>2</sub>- linkage. This is exploited in [[ChIP-on-chip]] transcriptomics experiments. Formaldehyde is also used as a denaturing agent in [[RNA]] gel [[electrophoresis]], preventing RNA from forming secondary structures. |
|||
====In photography==== |
|||
Formaldehyde is still used in low concentrations for [[C-41 process|process C-41]] (color negative film) stabilizer in the final wash step, as well as in the [[E-6 process|process E-6]] pre-bleach step, to obviate the need for it in the final wash. |
|||
====Tissue fixative and embalming agent==== |
|||
Formaldehyde solutions are used as a [[Fixation (histology)|fixative]] for [[microscopy]] and [[histology]]. Formaldehyde-based solutions are also used in [[embalming]] to disinfect and temporarily preserve human and animal remains. It is the ability of formaldehyde to fix the tissue that produces the tell-tale firmness of flesh in an embalmed body. Another use is called the "Sink Test" in post mortem examinations where the lungs of an animal are placed in an aqueous solution of fomaldehyde, if the lungs float it suggests the animal probably was breathing or able to breathe at the time of death. Several European countries restrict the use of formaldehyde, including the import of formaldehyde-treated products and embalming, and the European Union is considering a complete ban on formaldehyde usage (including embalming), subject to a review of List 4B of the Technical Annex to the Report from the Commission to the European Parliament and the Council on the Evaluation of the Active Substances of Plant Protection Products by the European Commission Services. Countries with a strong tradition of embalming corpses, such as Ireland and other colder-weather countries, have raised concerns. The European Union decided on September 22, 2007 to ban Formaldehyde use throughout Europe due to its carcinogenic properties.<ref>[http://www.webwire.com/ViewPressRel.asp?aId=41468 Formaldehyde Ban set for 22 Sept 2007<!-- Bot generated title -->]</ref> |
|||
==Interstellar formaldehyde== |
|||
{{Main|Interstellar formaldehyde}} |
|||
Formaldehyde was the first [[polyatomic]] [[Organic chemistry|organic]] molecule detected in the [[interstellar medium]]<ref>Zuckerman, B.; Buhl, D.; Palmer, P.; Snyder, L. E. 1970, ''Astrophysical Journal'', 160, 485</ref> and since its initial detection has been observed in many regions of the [[Milky Way galaxy|galaxy]]. Because of the widespread interest in interstellar formaldehyde it has recently been extensively studied, yielding new extragalactic sources.<ref name="mangum">J. G. Mangum ''et al''. 2008, ''Astrophysical Journal'', 673, 832.</ref>. A proposed mechanism for the formation is the hydrogenation of CO ice, shown below.<ref name="Woon">Woon, D.E. 2002, ''Astrophysical Journal'', 569, 541.</ref> |
|||
:H + CO --> HCO + H --> H<sub>2</sub>CO (rate constant=9.2*10<sup>-3</sup> s<sup>-1</sup>) |
|||
Formaldehyde appears to be a useful probe for astrochemists due to its low reactivity in the gas phase and to the fact that the 1<sub>10</sub> - 1<sub>11</sub> and 2<sub>11</sub> - 2<sub>12</sub> K-doublet transitions are rather clear. |
|||
== Safety == |
|||
Occupational exposure to formaldehyde by inhalation is mainly from three types of sources: [[Thermal decomposition|thermal]] or [[chemical decomposition]] of formaldehyde-based resins, formaldehyde emission from [[aqueous]] solutions (for example, embalming fluids), and the production of formaldehyde resulting from the [[combustion]] of a variety of organic compounds (for example, exhaust gases). Formaldehyde can be toxic, allergenic, and carcinogenic.<ref>IARC Press Release June 2004, http://www.iarc.fr/ENG/Press_Releases/archives/pr153a.html</ref> Because formaldehyde resins are used in many construction materials it is one of the more common indoor air pollutants.<ref>Indoor Air Pollution in California, Final Report, California Air Resources Board (2005) http://www.arb.ca.gov/research/indoor/ab1173/finalreport.htm, at pages 65 – 70.</ref> At concentrations above .1 ppm in air formaldehyde can irritate the eyes and [[mucous membrane]]s, resulting in watery eyes. <ref>Formaldehyde. OSHA Fact Sheet http://www.osha.gov/SLTC/formaldehyde/recognition.html</ref> Formaldehyde inhaled at this concentration may cause headaches, a burning sensation in the throat, and difficulty breathing, as well as triggering or aggravating asthma symptoms.<ref> http://www.oehha.ca.gov/air/chronic_rels/AllChrels.html California Office Of Health Hazard Assessment </ref><ref>Symptoms of Low-Level Formaldehyde Exposures, Health Canada, http://www.hc-sc.gc.ca/iyh-vsv/environ/formaldehyde_e.html</ref> |
|||
Formaldehyde is classified as a probable human carcinogen by the U.S. Environmental Protection Agency. The International Agency for Research on Cancer (IARC) has determined that there is "sufficient evidence" that occupational exposure to formaldehyde causes [[nasopharyngeal cancer]] in humans. <ref>http://monographs.iarc.fr/ENG/Monographs/vol88/volume88.pdf "Formaldehyde".</ref> |
|||
The [[United States Environmental Protection Agency]] (USEPA) allows no more than 16 ppb formaldehyde in the air in new buildings constructed for that agency.<ref> Testing for Indoor Air Quality, Baseline IAQ, and Materials, http://www.epa.gov/rtp/new-bldg/environmental/s_01445.htm</ref> On April 11th, 2008, FEMA announced that all trailers purchased by that agency in the future must meet the same standard.<ref> In the US, FEMA limits formaldehyde in trailers, Boston.com, referenced 9/4/2008[http://www.boston.com/news/nation/washington/articles/2008/04/11/fema_limits_formaldehyde_in_trailers/]</ref> |
|||
Formaldehyde can cause allergies and is part of the standard patch test series. People with formaldehyde allergy are advised to avoid [[formaldehyde releasers]] as well (''e.g.'', [[Quaternium-15]], [[imidazolidinyl urea]], and [[diazolidinyl urea]]).<ref>{{cite web | title = Formaldehyde allergy: What is formaldehyde and where is it found? | url = http://dermnetnz.org/dermatitis/formaldehyde-allergy.html | publisher = DermNet NZ}}</ref> Formaldehyde has been banned in cosmetics in both [[Sweden]] and [[Japan]].{{Fact|date=February 2008}} |
|||
===FEMA trailer incidents=== |
|||
====Hurricane Katrina & Rita==== |
|||
In the U.S. the [[Federal Emergency Management Agency]] (FEMA) provided travel trailers and mobile homes starting in 2006 for habitation by residents of the U.S. gulf coast displaced by [[Hurricane Katrina]] and [[Hurricane Rita]]. Some of the people who moved into the trailers complained of breathing difficulties, nosebleeds, and persistent headaches. Formaldehyde-catalyzed [[resins]] were used in the production of these homes. |
|||
The United States Centers For Disease Control and Prevention ([[CDC]]) performed indoor air quality testing for formaldehyde <ref>http://www.cdc.gov/niosh/nmam/pdfs/2016.pdf</ref> in some of the units. On February 14, 2008 the CDC announced that potentially hazardous levels of formaldehyde were found in many of the travel trailers and mobile homes provided by the agency.<ref>[http://www.cdc.gov/nceh/ehhe/trailerstudy/pdfs/SummaryofStudyFindings.pdf Formaldehyde Levels in FEMA-Supplied Trailers<!-- Bot generated title -->]</ref><ref>{{cite news | title = Are FEMA trailers ‘toxic tin cans’? | url = http://www.msnbc.msn.com/id/14011193/from/ET/#storyContinued | author = Mike Brunker | date = 2006-07-25 | publisher = [[MSNBC]]}}</ref> The CDC's preliminary evaluation of a scientifically established random sample of 519 travel trailers and mobile homes tested between Dec. 21, 2007 and Jan. 23, 2008 (2+ years after manufacture) showed average levels of formaldehyde in all units of about 77 parts per billion (ppb). Long-term exposure to levels in this range can be linked to an increased risk of cancer and, at levels above this range, there can also be a risk of respiratory illness. These levels are higher than expected in indoor air, where levels are commonly in the range of 10-20 ppb, and are higher than the Agency for Toxic Substance Disease Registry (ATSDR, division of the CDC) Minimal Risk Level (MRL) of 8 ppb <ref>[http://www.atsdr.cdc.gov/mrls/index.html ATSDR - Minimal Risk Levels for Hazardous Substances (MRLs)<!-- Bot generated title -->]</ref>. Levels measured ranged from 3 ppb to 590 ppb.<ref>[http://www.fema.gov/news/newsrelease.fema?id=42606 FEMA: CDC Releases Results Of Formaldehyde Level Tests<!-- Bot generated title -->]</ref> |
|||
The Federal Emergency Management Agency, which requested the testing by the CDC, said it would work aggressively to relocate all residents of the temporary housing as soon as possible. Lawsuits are being filed against FEMA as a result of the exposures.<ref>[http://www.washingtonpost.com/wp-dyn/content/article/2007/08/08/AR2007080801758.html Suit Filed Over FEMA Trailer Toxins - washingtonpost.com<!-- Bot generated title -->]</ref> |
|||
====Iowa Floods of 2008==== |
|||
Also in the U.S., problems arose in trailers again provided by FEMA to residents displaced by the [[Iowa Flood of 2008|Iowa floods of 2008]]. A couple months after moving to the trailers, occupants reported violent coughing, headaches, as well as [[Asthma]], [[Bronchitis]], and other problems. Tests showed that in some trailers, levels of formaldehyde exceeded the limits recommended by the U.S. [[Environmental Protection Agency]] and [[American Lung Association]].<ref>{{cite news | title = How We Tested for Formaldehyde | url = http://www.kgan.com/newsroom/top_stories/videos/kgan_vid_1515.shtml | author = Megan Terlecky | date = 2008-10-24 | publisher = [[KGAN-TV]]}}</ref>.<ref>{{cite news | title = FEMA disputes formaldehyde study of Iowa trailers| url = http://ap.google.com/article/ALeqM5jQTjw89qkEGgyRpUyURQLnyrPsMwD93V991G0 | author = NIGEL DUARA | date = 2008-10-21 | publisher = [[Associated Press]]}}</ref> The associated publicity has resulted in additional testing to begin in November.<ref>{{cite news | title = FEMA meets with mobile home residents over health concerns| url = http://www.gazetteonline.com/apps/pbcs.dll/article?AID=/20081025/NEWS/710259952/1006 | author = Cindy Hadish | date = 2008-10-24 | publisher = [[Cedar Rapids Gazette]]}}</ref> |
|||
==Contaminant in Food== |
|||
Scandals have broken in both [[2005 Indonesia food scare]] and [[2007 Vietnam food scare]] regarding the addition of formaldehyde to foods to extend shelf life. Foods known to be contaminated include noodles, salted fish, tofu, and rumors of chicken and beer. In humans, it is known to cause a number of detrimental effects including cancer. |
|||
== References == |
|||
<references/> |
|||
== External links == |
|||
* [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc02/icsc0275.htm International Chemical Safety Card 0275] (''gas'') |
|||
* [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc06/icsc0695.htm International Chemical Safety Card 0695] (''solution'') |
|||
* [http://monographs.iarc.fr/ENG/Monographs/vol88/volume88.pdf "Formaldehyde"], [[IARC]] Monograph |
|||
* [http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s089form.pdf Formaldehyde (gas)] from the 11th Report on Carcinogens of the [[U.S.]] [[National Toxicology Program]] (.pdf file) |
|||
* [http://www.npi.gov.au/database/substance-info/profiles/45.html Formaldehyde fact sheet] from the [[Australia]]n National Pollutant Inventory |
|||
* [http://www.cdc.gov/niosh/npg/npgd0293.html NIOSH Pocket Guide to Chemical Hazards] |
|||
* [http://www.kodak.com/global/en/service/Zmanuals/z131.shtml Process C-41 Using KODAK FLEXICOLOR Chemicals Publication Z-131] |
|||
* [http://www.formaldehyde.org/ Formaldehyde Council] |
|||
* [http://chemsub.online.fr?chemname=formaldehyde Formaldehyde Data Sheet], ChemSub Online |
|||
* [http://www.irsst.qc.ca/files/documents/PubIRSST/RG-473.pdf Prevention guide – Formaldehyde in the work place] |
|||
* [http://www.cdc.gov/niosh/topics/formaldehyde/ National Institute for Occupational Safety and Health - Formaldehyde Page] |
|||
[[Category:Aldehydes]] |
|||
[[Category:Carcinogens]] |
|||
[[Category:IARC Group 1 carcinogens]] |
|||
[[Category:Hazardous air pollutants]] |
|||
[[Category:Anatomical preservation]] |
|||
[[Category:Occupational safety and health]] |
|||
[[ar:فورمالدهيد]] |
|||
[[bg:Формалдехид]] |
|||
[[ca:Formaldehid]] |
|||
[[cs:Formaldehyd]] |
|||
[[da:Formaldehyd]] |
|||
[[de:Formaldehyd]] |
|||
[[et:Formaldehüüd]] |
|||
[[el:Φορμαλδεΰδη]] |
|||
[[es:Formaldehído]] |
|||
[[eo:Formaldehido]] |
|||
[[fa:فرمالدهید]] |
|||
[[fr:Méthanal]] |
|||
[[ko:폼알데하이드]] |
|||
[[id:Formaldehida]] |
|||
[[it:Formaldeide]] |
|||
[[he:פורמלין]] |
|||
[[kk:Формальдегид]] |
|||
[[la:Methanal]] |
|||
[[lv:Formaldehīds]] |
|||
[[lt:Formaldehidas]] |
|||
[[hu:Formaldehid]] |
|||
[[nl:Formaldehyde]] |
|||
[[ja:ホルムアルデヒド]] |
|||
[[no:Formaldehyd]] |
|||
[[pl:Aldehyd mrówkowy]] |
|||
[[pt:Metanal]] |
|||
[[ro:Formaldehidă]] |
|||
[[ru:Формальдегид]] |
|||
[[simple:Formaldehyde]] |
|||
[[sk:Formaldehyd]] |
|||
[[su:Formaldehida]] |
|||
[[fi:Formaldehydi]] |
|||
[[sv:Formaldehyd]] |
|||
[[te:ఫార్మాల్డిహైడ్]] |
|||
[[th:ฟอร์มาลดีไฮด์]] |
|||
[[vi:Fomanđêhít]] |
|||
[[tr:Formaldehit]] |
|||
[[uk:Формальдегід]] |
|||
[[zh:甲醛]] |
|||
Revision as of 03:38, 23 February 2009

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methanal
| |||
| Other names
formol, methyl aldehyde, methylene oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.002 | ||
| E number | E240 (preservatives) | ||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| CH2O | |||
| Molar mass | 30.03 g·mol−1 | ||
| Appearance | colorless gas | ||
| Density | 1 kg·m−3, gas | ||
| Melting point | -117 °C (156 K) | ||
| Boiling point | -19.3 °C (253.9 K) | ||
| > 100 g/100 ml (20 °C) | |||
| Structure | |||
| trigonal planar | |||
| 2.33168(1) D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, Flammable, Carcinogenic | ||
| NFPA 704 (fire diamond) | |||
| Flash point | -53 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
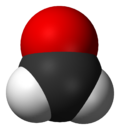
Formaldehyde (IUPAC name methanal) is a chemical compound with the formula H2CO. It is the simplest aldehyde. Formaldehyde exists in several forms aside from H2CO: the cyclic trimer trioxane and the polymer paraformaldehyde. It exists in water as the hydrate H2C(OH)2. Aqueous solutions of formaldehyde are referred to as formalin. "100%" formalin consists of a saturated solution of formaldehyde (roughly 40% by mass) in water, with a small amount of stabilizer, usually methanol to limit oxidation and polymerization. It is produced on a substantial scale of 6M tons/y. In view of its widespread use, toxicity, and volatility, exposure to formaldehyde is significant consideration for human health.[1]
Occurrence
Formaldehyde is an intermediate in the oxidation (or combustion) of methane as well as other carbon compounds, e.g. forest fires, in automobile exhaust, and in tobacco smoke. When produced in the atmosphere by the action of sunlight and oxygen on atmospheric methane and other hydrocarbons, it becomes part of smog.
Formaldehyde, as well as its oligomers and hydrates are rarely encountered in living organisms. Methanogenesis proceeds via the equivalent of formaldehyde, but this one-carbon species is masked as a methylene group in methanopterin. Formaldehyde is the primary cause of methanol's toxicity, since methanol is metabolised into toxic formaldehyde by alcohol dehydrogenase. Formaldehyde is converted to formic acid in the body. Formaldehyde has been detected in outer space (see below).
Synthesis and industrial production
Formaldehyde was first reported by the Russian chemist Aleksandr Butlerov (1828-1886), but was conclusively identified by August Wilhelm von Hofmann.[2]
Formaldehyde is produced industrially by the catalytic oxidation of methanol. The most common catalysts are silver metal or a mixture of an iron and molybdenum or vanadium oxides. In the more commonly used FORMOX process methanol and oxygen react at ca 250-400 °C in presence of iron oxide in combination with molybdenum and/or vanadium to produce formaldehyde according to the chemical equation:[1]
The silver-based catalyst is usually operated at a higher temperature, about 650 °C. Two chemical reactions on it simultaneously produce formaldehyde: that shown above and the dehydrogenation reaction:
- CH3OH → H2CO + H2
Formalin can be produced on a smaller scale using a whole range of other methods including conversion from ethanol instead of the normally-fed methanol feedstock. [citation needed] Such methods are of less commercial importance.
In principle formaldehyde could be generated by oxidation of methane, but this route is not industrially viable because the formaldehyde is more easily oxidized than methane.[1]
Organic chemistry
Formaldehyde is a building block in the synthesis of many other compounds of specialized and industrial significance. It exhibits most of the chemical properties of other aldehydes but is more reactive. For example it is more readily oxidized by atmospheric oxygen to formic acid (formic acid is found in ppm levels in commercial formaldehyde). Formaldehyde is a good electrophile, participating in electrophilic aromatic substitution reactions with aromatic compounds, and can undergo electrophilic addition reactions with alkenes and aromatics. Formaldehyde undergoes a Cannizzaro reaction in the presence of basic catalysts to produce formic acid and methanol.
Examples of organic synthetic applications
Condensation with acetaldehyde affords pentaerythritol, a chemical necessary in synthesizing PETN, a high explosive.[3] Condensation with phenols gives phenol-formaldehyde resins. With 4-substituted phenols one obtains calixarenes.[4]
When combined with hydrogen sulfide it forms trithiane.[5]
- 3 CH2O + 3 H2S → (CH2S)3 + 3 H2O
Industrial applications
Formaldehyde is a common building block for the synthesis of more complex compounds and materials. In approximate order of decreasing consumption, products generated from formaldehyde include urea formaldehyde resin, melamine resin, phenol formaldehyde resin, polyoxymethylene plastics, 1,4-butanediol, and methylene diphenyl diisocyanate.[1]
When reacted with phenol, urea, or melamine formaldehyde produces, respectively, hard thermoset phenol formaldehyde resin, urea formaldehyde resin, and melamine resin, which are commonly used in permanent adhesives such as those used in plywood or carpeting. It is used as the wet-strength resin added to sanitary paper products such as (listed in increasing concentrations injected into the paper machine headstock chest) facial tissue, table napkins, and roll towels. They are also foamed to make insulation, or cast into moulded products. Production of formaldehyde resins accounts for more than half of formaldehyde consumption.
Formaldehyde is also a precursor to polyfunctional alcohols such as pentaerythritol, which is used to make paints and explosives. Other formaldehyde derivatives include methylene diphenyl diisocyanate, an important component in polyurethane paints and foams, and hexamine, which is used in phenol-formaldehyde resins as well as the explosive RDX.
The textile industry uses formaldehyde-based resins as finishers to make fabrics crease-resistant.[6]
It is also used as an ingredient by some shampoo manufacturers.[citation needed]
Miscellaneous applications
Formaldehyde is a common component of many niche uses. Formaldehyde, along with 18 M (concentrated) sulfuric acid (the entire solution often called the Marquis reagent)[7], is used as an MDMA "testing kit" by such groups as Dancesafe as well as MDMA consumers. The solution alone cannot verify the presence of MDMA but reacts with many other chemicals that the MDMA tablet itself may be adulterated with. The reaction itself produces colors that correlate with these components.
As a disinfectant and biocide
An aqueous solution of formaldehyde can be useful as a disinfectant as it kills most bacteria and fungi (including their spores). It is also used as a preservative in vaccines. Formaldehyde solutions are applied topically in medicine to dry the skin, such as in the treatment of warts. Many aquarists use formaldehyde as a treatment for the parasites ichthyophthirius and Cryptocaryon irritans.[8]
Formaldehyde preserves or fixes tissue or cells by irreversibly cross-linking primary amino groups in proteins with other nearby nitrogen atoms in protein or DNA through a -CH2- linkage. This is exploited in ChIP-on-chip transcriptomics experiments. Formaldehyde is also used as a denaturing agent in RNA gel electrophoresis, preventing RNA from forming secondary structures.
In photography
Formaldehyde is still used in low concentrations for process C-41 (color negative film) stabilizer in the final wash step, as well as in the process E-6 pre-bleach step, to obviate the need for it in the final wash.
Tissue fixative and embalming agent
Formaldehyde solutions are used as a fixative for microscopy and histology. Formaldehyde-based solutions are also used in embalming to disinfect and temporarily preserve human and animal remains. It is the ability of formaldehyde to fix the tissue that produces the tell-tale firmness of flesh in an embalmed body. Another use is called the "Sink Test" in post mortem examinations where the lungs of an animal are placed in an aqueous solution of fomaldehyde, if the lungs float it suggests the animal probably was breathing or able to breathe at the time of death. Several European countries restrict the use of formaldehyde, including the import of formaldehyde-treated products and embalming, and the European Union is considering a complete ban on formaldehyde usage (including embalming), subject to a review of List 4B of the Technical Annex to the Report from the Commission to the European Parliament and the Council on the Evaluation of the Active Substances of Plant Protection Products by the European Commission Services. Countries with a strong tradition of embalming corpses, such as Ireland and other colder-weather countries, have raised concerns. The European Union decided on September 22, 2007 to ban Formaldehyde use throughout Europe due to its carcinogenic properties.[9]
Interstellar formaldehyde
Formaldehyde was the first polyatomic organic molecule detected in the interstellar medium[10] and since its initial detection has been observed in many regions of the galaxy. Because of the widespread interest in interstellar formaldehyde it has recently been extensively studied, yielding new extragalactic sources.[11]. A proposed mechanism for the formation is the hydrogenation of CO ice, shown below.[12]
- H + CO --> HCO + H --> H2CO (rate constant=9.2*10-3 s-1)
Formaldehyde appears to be a useful probe for astrochemists due to its low reactivity in the gas phase and to the fact that the 110 - 111 and 211 - 212 K-doublet transitions are rather clear.
Safety
Occupational exposure to formaldehyde by inhalation is mainly from three types of sources: thermal or chemical decomposition of formaldehyde-based resins, formaldehyde emission from aqueous solutions (for example, embalming fluids), and the production of formaldehyde resulting from the combustion of a variety of organic compounds (for example, exhaust gases). Formaldehyde can be toxic, allergenic, and carcinogenic.[13] Because formaldehyde resins are used in many construction materials it is one of the more common indoor air pollutants.[14] At concentrations above .1 ppm in air formaldehyde can irritate the eyes and mucous membranes, resulting in watery eyes. [15] Formaldehyde inhaled at this concentration may cause headaches, a burning sensation in the throat, and difficulty breathing, as well as triggering or aggravating asthma symptoms.[16][17]
Formaldehyde is classified as a probable human carcinogen by the U.S. Environmental Protection Agency. The International Agency for Research on Cancer (IARC) has determined that there is "sufficient evidence" that occupational exposure to formaldehyde causes nasopharyngeal cancer in humans. [18] The United States Environmental Protection Agency (USEPA) allows no more than 16 ppb formaldehyde in the air in new buildings constructed for that agency.[19] On April 11th, 2008, FEMA announced that all trailers purchased by that agency in the future must meet the same standard.[20]
Formaldehyde can cause allergies and is part of the standard patch test series. People with formaldehyde allergy are advised to avoid formaldehyde releasers as well (e.g., Quaternium-15, imidazolidinyl urea, and diazolidinyl urea).[21] Formaldehyde has been banned in cosmetics in both Sweden and Japan.[citation needed]
FEMA trailer incidents
Hurricane Katrina & Rita
In the U.S. the Federal Emergency Management Agency (FEMA) provided travel trailers and mobile homes starting in 2006 for habitation by residents of the U.S. gulf coast displaced by Hurricane Katrina and Hurricane Rita. Some of the people who moved into the trailers complained of breathing difficulties, nosebleeds, and persistent headaches. Formaldehyde-catalyzed resins were used in the production of these homes.
The United States Centers For Disease Control and Prevention (CDC) performed indoor air quality testing for formaldehyde [22] in some of the units. On February 14, 2008 the CDC announced that potentially hazardous levels of formaldehyde were found in many of the travel trailers and mobile homes provided by the agency.[23][24] The CDC's preliminary evaluation of a scientifically established random sample of 519 travel trailers and mobile homes tested between Dec. 21, 2007 and Jan. 23, 2008 (2+ years after manufacture) showed average levels of formaldehyde in all units of about 77 parts per billion (ppb). Long-term exposure to levels in this range can be linked to an increased risk of cancer and, at levels above this range, there can also be a risk of respiratory illness. These levels are higher than expected in indoor air, where levels are commonly in the range of 10-20 ppb, and are higher than the Agency for Toxic Substance Disease Registry (ATSDR, division of the CDC) Minimal Risk Level (MRL) of 8 ppb [25]. Levels measured ranged from 3 ppb to 590 ppb.[26]
The Federal Emergency Management Agency, which requested the testing by the CDC, said it would work aggressively to relocate all residents of the temporary housing as soon as possible. Lawsuits are being filed against FEMA as a result of the exposures.[27]
Iowa Floods of 2008
Also in the U.S., problems arose in trailers again provided by FEMA to residents displaced by the Iowa floods of 2008. A couple months after moving to the trailers, occupants reported violent coughing, headaches, as well as Asthma, Bronchitis, and other problems. Tests showed that in some trailers, levels of formaldehyde exceeded the limits recommended by the U.S. Environmental Protection Agency and American Lung Association.[28].[29] The associated publicity has resulted in additional testing to begin in November.[30]
Contaminant in Food
Scandals have broken in both 2005 Indonesia food scare and 2007 Vietnam food scare regarding the addition of formaldehyde to foods to extend shelf life. Foods known to be contaminated include noodles, salted fish, tofu, and rumors of chicken and beer. In humans, it is known to cause a number of detrimental effects including cancer.
References
- ^ a b c d G. Reuss, W. Disteldorf, A. O. Gamer, A. Hilt, “Formaldehyde” in Ullmann’s Encyclopedia of Industrial Chemistry Wiley-VCH, 2005, Weinheim.
- ^ J Read, Text-Book of Organic Chemistry, G Bell & Sons, London, 1935
- ^ H. B. J. Schurink (1941). "Pentaerythritol". Organic Syntheses. 1: 425; Collected Volumes, vol. 1.
- ^ Gutsche, C. D.; Iqbal, M. (1993). "p-tert-Butylcalix[4]arene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 75. - ^ Bost, R. W.; Constable, E. W. (1943). "sym-Trithiane". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 2, p. 610. - ^ FORMALDEHYDE IN CLOTHING AND OTHER TEXTILES
- ^ DanceSafe: testing kit info
- ^ University of Florida - Use of Formalin to Control Fish Parasites http://edis.ifas.ufl.edu/VM061
- ^ Formaldehyde Ban set for 22 Sept 2007
- ^ Zuckerman, B.; Buhl, D.; Palmer, P.; Snyder, L. E. 1970, Astrophysical Journal, 160, 485
- ^ J. G. Mangum et al. 2008, Astrophysical Journal, 673, 832.
- ^ Woon, D.E. 2002, Astrophysical Journal, 569, 541.
- ^ IARC Press Release June 2004, http://www.iarc.fr/ENG/Press_Releases/archives/pr153a.html
- ^ Indoor Air Pollution in California, Final Report, California Air Resources Board (2005) http://www.arb.ca.gov/research/indoor/ab1173/finalreport.htm, at pages 65 – 70.
- ^ Formaldehyde. OSHA Fact Sheet http://www.osha.gov/SLTC/formaldehyde/recognition.html
- ^ http://www.oehha.ca.gov/air/chronic_rels/AllChrels.html California Office Of Health Hazard Assessment
- ^ Symptoms of Low-Level Formaldehyde Exposures, Health Canada, http://www.hc-sc.gc.ca/iyh-vsv/environ/formaldehyde_e.html
- ^ http://monographs.iarc.fr/ENG/Monographs/vol88/volume88.pdf "Formaldehyde".
- ^ Testing for Indoor Air Quality, Baseline IAQ, and Materials, http://www.epa.gov/rtp/new-bldg/environmental/s_01445.htm
- ^ In the US, FEMA limits formaldehyde in trailers, Boston.com, referenced 9/4/2008[1]
- ^ "Formaldehyde allergy: What is formaldehyde and where is it found?". DermNet NZ.
- ^ http://www.cdc.gov/niosh/nmam/pdfs/2016.pdf
- ^ Formaldehyde Levels in FEMA-Supplied Trailers
- ^ Mike Brunker (2006-07-25). "Are FEMA trailers 'toxic tin cans'?". MSNBC.
- ^ ATSDR - Minimal Risk Levels for Hazardous Substances (MRLs)
- ^ FEMA: CDC Releases Results Of Formaldehyde Level Tests
- ^ Suit Filed Over FEMA Trailer Toxins - washingtonpost.com
- ^ Megan Terlecky (2008-10-24). "How We Tested for Formaldehyde". KGAN-TV.
- ^ NIGEL DUARA (2008-10-21). "FEMA disputes formaldehyde study of Iowa trailers". Associated Press.
- ^ Cindy Hadish (2008-10-24). "FEMA meets with mobile home residents over health concerns". Cedar Rapids Gazette.
External links
- International Chemical Safety Card 0275 (gas)
- International Chemical Safety Card 0695 (solution)
- "Formaldehyde", IARC Monograph
- Formaldehyde (gas) from the 11th Report on Carcinogens of the U.S. National Toxicology Program (.pdf file)
- Formaldehyde fact sheet from the Australian National Pollutant Inventory
- NIOSH Pocket Guide to Chemical Hazards
- Process C-41 Using KODAK FLEXICOLOR Chemicals Publication Z-131
- Formaldehyde Council
- Formaldehyde Data Sheet, ChemSub Online
- Prevention guide – Formaldehyde in the work place
- National Institute for Occupational Safety and Health - Formaldehyde Page