Boron nitride: Difference between revisions
No edit summary |
various fixes; to be continued; properties summarized in table |
||
| Line 21: | Line 21: | ||
| MolarMass = 24.818 g/mol |
| MolarMass = 24.818 g/mol |
||
| Appearance = white solid |
| Appearance = white solid |
||
| Density = |
| Density = |
||
| MeltingPt = 2500 °C |
| MeltingPt = 2500 °C |
||
| Melting_notes = (sublimes) |
| Melting_notes = (sublimes) |
||
| Line 34: | Line 34: | ||
| pKa = |
| pKa = |
||
| pKb = |
| pKb = |
||
| BandGap = |
| BandGap = |
||
| ElectronMobility = 200 cm<sup>2</sup>/(V*s) (cubic) |
| ElectronMobility = 200 cm<sup>2</sup>/(V*s) (cubic) |
||
| ThermalConductivity = |
| ThermalConductivity = |
||
| RefractIndex = |
| RefractIndex = |
||
}} |
}} |
||
| Section3 = {{Chembox Structure |
| Section3 = {{Chembox Structure |
||
| Line 92: | Line 92: | ||
| OtherCpds = }} |
| OtherCpds = }} |
||
}} |
}} |
||
'''Boron nitride''' ('''BN''') is a [[binary compound|binary chemical compound]], consisting of equal numbers of [[boron]] and [[nitrogen]] atoms. Its [[empirical formula]] is therefore BN. Boron nitride is [[isoelectronic]] with [[carbon]] and, like carbon, boron nitrides exists as various [[Polymorphism (materials science)|polymorphic forms]]. The [[hexagonal]] form corresponds to [[graphite]] - it is most stable, soft ([[Mohs scale of mineral hardness|Mohs hardness]] ~ 2), and is therefore used a lubricant. The [[zinc blende]] form is analogous to [[diamond]] and is one of the hardest materials; and the rare [[wurtzite]] modification is similar to [[lonsdaleite]] <ref>Silberberg, Martin S. ''Chemistry: The Molecular Nature of Matter and Change, Fifth Edition.'' New York: McGraw-Hill, 2009. p. 483</ref> and |
'''Boron nitride''' ('''BN''') is a [[binary compound|binary chemical compound]], consisting of equal numbers of [[boron]] and [[nitrogen]] atoms. Its [[empirical formula]] is therefore BN. Boron nitride is [[isoelectronic]] with [[carbon]] and, like carbon, boron nitrides exists as various [[Polymorphism (materials science)|polymorphic forms]]. The [[hexagonal]] form corresponds to [[graphite]] - it is most stable, soft ([[Mohs scale of mineral hardness|Mohs hardness]] ~ 2), and is therefore used a lubricant. The cubic [[zinc blende]] form is analogous to [[diamond]] and is one of the hardest materials; and the rare [[wurtzite]] modification is similar to [[lonsdaleite]] <ref>Silberberg, Martin S. ''Chemistry: The Molecular Nature of Matter and Change, Fifth Edition.'' New York: McGraw-Hill, 2009. p. 483</ref> and may be even harder than the cubic form. |
||
==Hexagonal BN== |
==Hexagonal BN== |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
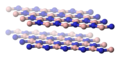
The [[graphite]]-like polymorph of boron nitride, known as hexagonal boron nitride, h-BN, α-BN, or g-BN (graphitic BN), and sometimes called "white graphite," is the most widely used polymorph.<ref>Jochen Greim, Karl A. Schwetz “Boron Carbide, Boron Nitride, and Metal Borides” in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH: Weinheim: 2005. DOI: 10.1002/14356007.a04_295.pub2</ref> The hexagonal polymorph is composed of layers of hexagonal sheets, analogous to graphite. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms laying over and above nitrogen atoms. This registry reflects the polarity of the B-N bonds. The diminished covalency in BN results in diminished electrical conductivity relative to graphite, which is a [[semimetal]] that conducts electricity through a network of pi-bonds in the plane of its hexagonal sheets. The diminished electron-delocalizaton in hexagonal-BN is indicated by its absence of color, which signals a large [[band gap]]. |
The [[graphite]]-like polymorph of boron nitride, known as hexagonal boron nitride, h-BN, α-BN, or g-BN (graphitic BN), and sometimes called "white graphite," is the most widely used polymorph.<ref>Jochen Greim, Karl A. Schwetz “Boron Carbide, Boron Nitride, and Metal Borides” in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH: Weinheim: 2005. DOI: 10.1002/14356007.a04_295.pub2</ref> The hexagonal polymorph is composed of layers of hexagonal sheets, analogous to graphite. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms laying over and above nitrogen atoms. This registry reflects the polarity of the B-N bonds. The diminished covalency in BN results in diminished electrical conductivity relative to graphite, which is a [[semimetal]] that conducts electricity through a network of pi-bonds in the plane of its hexagonal sheets. The diminished electron-delocalizaton in hexagonal-BN is indicated by its absence of color, which signals a large [[band gap]]. |
||
| Line 113: | Line 119: | ||
Combustion of boron powder in nitrogen [[plasma (physics)|plasma]] at 5500 °C yields [[Ultrafine particles|ultrafine]] boron nitride for lubricants and [[toner]]s. |
Combustion of boron powder in nitrogen [[plasma (physics)|plasma]] at 5500 °C yields [[Ultrafine particles|ultrafine]] boron nitride for lubricants and [[toner]]s. |
||
| ⚫ | |||
| ⚫ | |||
Image:Boron-nitride-(hexagonal)-top-3D-balls.png|<center>α-BN, hexagonal</center> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
==Cubic boron nitride== |
==Cubic boron nitride== |
||
| Line 130: | Line 129: | ||
Low-pressure deposition of thin films of cubic boron nitride is possible. For selective etching of the deposited hexagonal phase during [[chemical vapor deposition]], [[boron trifluoride]] is used (''cf.'' use of atomic hydrogen for selective etching of graphite during deposition of diamond films). [[Ion beam deposition]], [[Plasma Enhanced CVD]], [[pulsed laser deposition]], [[reactive sputtering]], and other [[physical vapor deposition]] methods are used as well. |
Low-pressure deposition of thin films of cubic boron nitride is possible. For selective etching of the deposited hexagonal phase during [[chemical vapor deposition]], [[boron trifluoride]] is used (''cf.'' use of atomic hydrogen for selective etching of graphite during deposition of diamond films). [[Ion beam deposition]], [[Plasma Enhanced CVD]], [[pulsed laser deposition]], [[reactive sputtering]], and other [[physical vapor deposition]] methods are used as well. |
||
==Properties== |
|||
Properties of BN at room temperature<ref>{{cite web| url= http://www.ioffe.ru/SVA/NSM/Semicond/BN/index.html| title=Ioffe database| accessdate=2009-05-05}}</ref> |
|||
{| class="wikitable" align="center" |
|||
|- |
|||
!BN phase |
|||
![[Hexagonal]] |
|||
![[zinc blende|cubic |
|||
![[wurtzite |
|||
|- |
|||
!Density (g/cm<sup>3</sup>) |
|||
|2.18 |
|||
|3.45 |
|||
|3.49 |
|||
|- |
|||
![[Bulk modulus]] (GPa) |
|||
|36.5 |
|||
|400 |
|||
|400 |
|||
|- |
|||
![[Thermal conductivity]] (W/cm K) |
|||
|6ǁ; 0.3 ┴ |
|||
|7.4 |
|||
| |
|||
|- |
|||
![[Bandgap]] (eV) |
|||
|5.2 |
|||
|6.4 |
|||
|4.5-5.5 |
|||
|- |
|||
![[Refractive index]] |
|||
|1.8 |
|||
|2.1 |
|||
|2.05 |
|||
|} |
|||
==Other polymorphs of BN== |
==Other polymorphs of BN== |
||
Revision as of 08:22, 8 May 2009
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.030.111 |
| EC Number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| BN | |
| Molar mass | 24.818 g/mol |
| Appearance | white solid |
| Melting point | 2500 °C |
| insoluble | |
| Solubility | insoluble in acids |
| Electron mobility | 200 cm2/(V*s) (cubic) |
| Structure | |
| hexagonal, zinc clende, wurzite, etc. | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
14.77 J mol−1 K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
476.98 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−250.91 kJ mol−1 |
| Related compounds | |
Other anions
|
BP, BAs B4C, B2O3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Boron nitride (BN) is a binary chemical compound, consisting of equal numbers of boron and nitrogen atoms. Its empirical formula is therefore BN. Boron nitride is isoelectronic with carbon and, like carbon, boron nitrides exists as various polymorphic forms. The hexagonal form corresponds to graphite - it is most stable, soft (Mohs hardness ~ 2), and is therefore used a lubricant. The cubic zinc blende form is analogous to diamond and is one of the hardest materials; and the rare wurtzite modification is similar to lonsdaleite [1] and may be even harder than the cubic form.
Hexagonal BN
-
α-BN, hexagonal -
β-BN, sphalerite structure -
BN, wurtzite structure
The graphite-like polymorph of boron nitride, known as hexagonal boron nitride, h-BN, α-BN, or g-BN (graphitic BN), and sometimes called "white graphite," is the most widely used polymorph.[2] The hexagonal polymorph is composed of layers of hexagonal sheets, analogous to graphite. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms laying over and above nitrogen atoms. This registry reflects the polarity of the B-N bonds. The diminished covalency in BN results in diminished electrical conductivity relative to graphite, which is a semimetal that conducts electricity through a network of pi-bonds in the plane of its hexagonal sheets. The diminished electron-delocalizaton in hexagonal-BN is indicated by its absence of color, which signals a large band gap.
Hexagonal BN is a lubricant at both low and high temperatures (up to 900 °C, even in oxidizing atmosphere). It is particularly useful lubricant in situations where the electrical conductivity or chemical reactivity of graphite would be problematic. Since the lubricity mechanism does not involve water molecules trapped between the layers, boron nitride lubricants can be used even in vacuum, e.g. for space applications.
Hexagonal boron nitride is stable in temperatures up to 1000 °C in air, 1400 °C in vacuum, and 2800 °C in an inert atmosphere. It has one of the best thermal conductivities of all electric insulators. It is fairly chemically inert and is not wetted by many melted materials (e.g. aluminium, copper, zinc, iron and steels, germanium, silicon, boron, cryolite, glass and halide salts).[citation needed]
Fine-grained h-BN is used in some cosmetics, paints, dental cements, and pencil leads.[citation needed]
Preparation of hexagonal BN
Hexagonal boron nitride is produced by the nitridation or ammonolysis of boron trioxide. h-BN parts can be made by hot-pressing with subsequent machining; due to the mechanical hardness similar to graphite, the machining cost is low. The parts are made from boron nitride powders, using boron oxide as a sintering agent. Thin films of boron nitride can be obtained by chemical vapor deposition from boron trichloride and nitrogen precursors. Industrial production is based on two reactions: melted boric acid with ammonia, and boric acid or alkaline borates with urea, guanidine, melamin, or other suitable organic nitrogen compounds in nitrogen atmosphere. The synthesis reactions involving ammonia and boric acid are shown below:
- B(OH)
3(s) + 3NH
3(g) → B(NH
2)
3(s) + 3H
2O(g)
And by heating:
- B(NH
2)
3(s) → 2NH
3(g) + BN(s)
Combustion of boron powder in nitrogen plasma at 5500 °C yields ultrafine boron nitride for lubricants and toners.
Cubic boron nitride
Cubic boron nitride is extremely hard, although less so than diamond and some related materials. Also like diamond, cubic boron nitride is an electrical insulator and an excellent conductor of heat. This diamond-like polymorph, known as cubic boron nitride, c-BN, β-BN, or z-BN (after zinc blende crystalline structure), is widely used as an abrasive for industrial tools[3]. Its usefulness arises from its insolubility in iron, nickel, and related alloys at high temperatures, whereas diamond is soluble in these metals to give carbides. Polycrystalline c-BN abrasives are therefore used for machining steel, whereas diamond abrasives are preferred for aluminium alloys, ceramics, and stone. Like diamond, cubic BN has good thermal conductivity, caused by phonons. In contact with oxygen at high temperatures, BN forms a passivation layer of boron oxide. Boron nitride binds well with metals, due to formation of interlayers of metal borides or nitrides. Materials with cubic boron nitride crystals are often used in the tool bits of cutting tools. For grinding applications, softer binders, e.g. resin, porous ceramics, and soft metals, are used. Ceramic binders can be used as well. Commercial products are known under names "Borazon" (by Diamond Innovations), and "Elbor" or "Cubonite" (by Russian vendors).
Sintered cubic boron nitride is an electrically insulating heatsink material of potential value in microelectronics.
Preparation of cubic BN
Cubic boron nitride is produced by treating hexagonal boron nitride at high pressure and temperature, much as synthetic diamond is produced from graphite. Direct conversion of hexagonal boron nitride to the cubic form occurs at pressures up to 18 GPa and temperatures between 1730-3230 °C; addition of small amount of boron oxide can lower the required pressure to 4-7 GPa and temperature to 1500 °C. Industrially, BN conversion using catalysts is used instead; the catalyst materials differ for different production methods, eg. lithium, potassium, or magnesium, their nitrides, their fluoronitrides, water with ammonium compounds, or hydrazine. Other industrial synthesis methods use crystal growth in temperature gradient, or explosive shock wave. The shock wave method is used to produce material called heterodiamond, a superhard compound of boron, carbon, and nitrogen.
Low-pressure deposition of thin films of cubic boron nitride is possible. For selective etching of the deposited hexagonal phase during chemical vapor deposition, boron trifluoride is used (cf. use of atomic hydrogen for selective etching of graphite during deposition of diamond films). Ion beam deposition, Plasma Enhanced CVD, pulsed laser deposition, reactive sputtering, and other physical vapor deposition methods are used as well.
Properties
Properties of BN at room temperature[4]
| BN phase | Hexagonal | [[zinc blende|cubic | [[wurtzite |
|---|---|---|---|
| Density (g/cm3) | 2.18 | 3.45 | 3.49 |
| Bulk modulus (GPa) | 36.5 | 400 | 400 |
| Thermal conductivity (W/cm K) | 6ǁ; 0.3 ┴ | 7.4 | |
| Bandgap (eV) | 5.2 | 6.4 | 4.5-5.5 |
| Refractive index | 1.8 | 2.1 | 2.05 |
Other polymorphs of BN
w-BN
Known as w-BN, hexagonal boron nitride is a superhard phase that occurs at high pressures. This hexagonal phase differs from the layered graphitic material: it adopts the wurtzite structure, giving it a greater indentation strength than diamond.[5]
Rhombohedral boron nitride
Rhombohedral boron nitride is similar to hexagonal boron nitride. It is formed transitionally during conversion of cubic BN to hexagonal form.
Boron nitride fibers
Hexagonal BN can be prepared in the form of fibers, structurally similar to carbon fibers, sometimes called "white carbon fiber." They can be prepared by thermal decomposition of extruded borazine fibers with addition of boron oxide in nitrogen at 1800 °C. The material also arises by the thermal decomposition of cellulose fibers impregnated with boric acid or ammonium tetraborate in an atmosphere of ammonia and nitrogen above 1000 °C. Boron nitride fibers are used as reinforcement in composite materials, with the matrix materials ranging from organic resins to ceramics to metals (see Metal matrix composites).
Nanostructured BN
Boron nitride nanotubes
Like BN fibers, boron nitride nanotubes (BNNTs) show promise for aerospace applications where integration of boron and in particular the light isotope of boron (10B) into structural materials improves their radiation-shielding properties, due to 10B's neutron absorption properties. Such 10BN materials are of particular theoretical value as composite structural material in future manned interplanetary spacecraft, where absorption-shielding from cosmic ray spallation neutrons is expected to be a particular asset in light construction materials.[6].
Boron nitride nanomesh
Boron nitride nanomesh is a new inorganic nanostructured two-dimensional material. It consists of a single layer of boron (B) and nitrogen (N) atoms, which forms by self-assembly a highly regular mesh after high-temperature exposure of a clean rhodium[7] or ruthenium[8] surface to borazine under ultra-high vacuum. The nanomesh looks like an assembly of hexagonal pores. The distance between 2 pore centers is 3.2 nm and the pore diameter is ~2 nm.
The boron nitride nanomesh is not only stable under vacuum[7], air[9] and some liquids[10][11], but also up to temperatures of 796oC (1070 K)[7]. In addition it shows the extraordinary ability to trap molecules[10] and metallic clusters[8], which have similar sizes to the nanomesh pores, forming a well-ordered array. These characteristics promise interesting applications of the nanomesh in areas like nanocatalysis, surface functionalisation, spintronics, quantum computing and data storage media like hard drives.
Amorphous boron nitride
Layers of amorphous boron nitride (a-BN) are used in some semiconductor devices, e. g. MISFETs. They can be prepared by chemical decomposition of trichloroborazine with caesium, or by thermal chemical vapor deposition methods. Thermal CVD can be also used for deposition of h-BN layers, or at high temperatures, c-BN [12].
Composites containing BN
Addition of boron nitride to silicon nitride ceramics improves the thermal shock resistance of the resulting material. For the same purpose, BN is added also to silicon nitride-alumina and titanium nitride-alumina ceramics. Other materials being reinforced with BN are e.g. alumina and zirconia, borosilicate glasses, glass ceramics, enamels, and composite ceramics with titanium boride-boron nitride and titanium boride-aluminium nitride-boron nitride and silicon carbide-boron nitride composition.
Due to its excellent dielectric and thermal properties, BN is used in electronics e.g. as a substrate for semiconductors, microwave-transparent windows, structural material for seals, electrodes and catalyst carriers in fuel cells and batteries.
h-BN can be included in ceramics, alloys, resins, plastics, rubbers and other materials, giving them self-lubricating properties. Such materials are suitable for construction of e.g. bearings. Plastics filled with BN have decreased thermal expansion, increased thermal conductivity, increased electrical insulation properties, and cause reduced wear to adjacent parts.
See also
Notes
- ^ Silberberg, Martin S. Chemistry: The Molecular Nature of Matter and Change, Fifth Edition. New York: McGraw-Hill, 2009. p. 483
- ^ Jochen Greim, Karl A. Schwetz “Boron Carbide, Boron Nitride, and Metal Borides” in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH: Weinheim: 2005. DOI: 10.1002/14356007.a04_295.pub2
- ^ Tool coatings-new developments for forming, cutting and abrasive machining. Gaebler, J.; Bewilogua, K.; Biehl, S.; Brand, J.; Hoefer, M.; Keunecke, M.; Schaefer, L.; Thomsen, H.; Weber, M. Fraunhofer-Institut fuer Schicht- und Oberflaechentechnik IST, Braunschweig, Germany. Annual Technical Conference Proceedings - Society of Vacuum Coaters (2007), 50th 608-615. Publisher: Society of Vacuum Coaters
- ^ "Ioffe database". Retrieved 2009-05-05.
- ^ http://www.physorg.com/news153658987.html
- ^ ANU - Nanotube Research
- ^ a b c M. Corso; et al. (2004). "Boron Nitride Nanomesh". Science. 303: 217–220. doi:10.1126/science.1091979.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b A. Goriachko; et al. (2007). "Self-assembly of a hexagonal boron nitride nanomesh on Ru(0001)". Langmuir Lett. 23: 2928–2931. doi:10.1021/la062990t.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ O. Bunk; et al. (2007). "Surface X-ray diffraction study of boron-nitride nanomesh in air". Surf. Sci. 601: L7–L10. doi:10.1016/j.susc.2006.11.018.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b S. Berner, M. Corso; et al. (2007). "Boron Nitride Nanomesh: Functionality from a Corrugated Monolayer". Angew. Chem. Int. Ed. 46: 5115–5119. doi:10.1002/anie.200700234.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ R. Widmer; et al. (2007). "Electrolytic in situ STM investigation of h-BN-Nanomesh". Electrochem. Comm. 9: 2484–2488. doi:10.1016/j.elecom.2007.07.019.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ W. Schmolla "Positive drift effect of BN-InP enhancement n-channel MISFET" International Journal of Electronics 58 (1985) 35
This article needs additional citations for verification. (January 2008) |
External links
- Database of boron nitride properties
- National Pollutant Inventory: Boron and Compounds
- Fiz Chemie Berlin thermophysical database
- Materials Safety Data Sheet at University of Oxford