Α-Ketobutyric acid: Difference between revisions
Appearance
Content deleted Content added
Script assisted update of chemical identifiers from ChemSpider for the Chem/Drugbox validation project. |
image |
||
| Line 5: | Line 5: | ||
|Name=α-Ketobutyric acid |
|Name=α-Ketobutyric acid |
||
|ImageFile=Alpha-ketobutyric acid.svg |
|ImageFile=Alpha-ketobutyric acid.svg |
||
|ImageSize= |
|||
|ImageFile1=Alpha-Ketobutyric acid.png |
|||
|ImageSize= |
|ImageSize= |
||
|IUPACName=2-oxobutanoic acid |
|IUPACName=2-oxobutanoic acid |
||
Revision as of 06:47, 27 January 2010
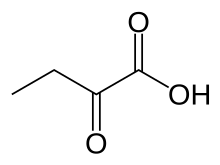
| |

| |
| Names | |
|---|---|
| IUPAC name
2-oxobutanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.080 |
| MeSH | Alpha-ketobutyric+acid |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O3 | |
| Molar mass | 102.089 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
α-Ketobutyric acid is a product of the lysis of cystathionine.
It is also one of the degradation products of threonine.
It can be converted to propionyl-CoA (and subsequently methylmalonyl CoA, which can be converted to succinyl CoA, a CAC intermediate), and thus enter the citric acid cycle.
See also
