Esophageal cancer: Difference between revisions
No edit summary |
Reverted to revision 424081126 by Chaojoker; largely unhelpful and ungrammatical changes - please compare with other medical articles - also removed odd section about drugs. (TW) |
||
| Line 14: | Line 14: | ||
| MeshID = D004938 |
| MeshID = D004938 |
||
}} |
}} |
||
'''Esophageal cancer''' (or '''oesophageal cancer''') is [[cancer|malignancy]] |
'''Esophageal cancer''' (or '''oesophageal cancer''') is [[cancer|malignancy]] of the [[esophagus]]. There are various subtypes, primarily [[squamous]] cell cancer (approx 90-95% of all esophageal cancer worldwide) and [[adenocarcinoma]] (approx. 50-80% of all esophageal cancer in the United States). Squamous cell cancer arises from the cells that line the upper part of the esophagus. Adenocarcinoma arises from glandular cells that are present at the junction of the esophagus and stomach.<ref>[http://www.mountsinai.org/Other/Diseases/Esophageal%20cancer Esophageal cancer] at [[Mount Sinai Hospital, New York|Mount Sinai Hospital]]</ref> |
||
Esophageal tumors usually lead to [[dysphagia]] (difficulty [[swallowing]]), pain and other symptoms, and are diagnosed with [[biopsy]]. Small and localized tumors are treated [[surgery|surgically]] with [[curative care|curative]] intent. Larger tumors tend not to be operable and hence are treated with [[palliative care]]; their growth can still be delayed with [[chemotherapy]], [[radiation therapy|radiotherapy]] or a combination of the two. In some cases chemo- and radiotherapy can render these larger tumors operable |
Esophageal tumors usually lead to [[dysphagia]] (difficulty [[swallowing]]), pain and other symptoms, and are diagnosed with [[biopsy]]. Small and localized tumors are treated [[surgery|surgically]] with [[curative care|curative]] intent. Larger tumors tend not to be operable and hence are treated with [[palliative care]]; their growth can still be delayed with [[chemotherapy]], [[radiation therapy|radiotherapy]] or a combination of the two. In some cases chemo- and radiotherapy can render these larger tumors operable. Prognosis depends on the extent of the disease and other medical problems, but is fairly poor.<ref name=Enzinger>{{cite journal |author=Enzinger PC, Mayer RJ |title=Esophageal cancer |journal=N. Engl. J. Med. |volume=349 |issue=23 |pages=2241–52 |year=2003 |pmid=14657432 |doi=10.1056/NEJMra035010}}</ref> |
||
==Classification== |
==Classification== |
||
[[Image:Esophageal adenocarcinoma - intermed mag.jpg|right|thumb|[[Micrograph]] of an esophageal [[adenocarcinoma]] (dark blue - upper-left of image) and normal squamous epithelium (upper-right of image). [[H&E stain]].]] |
[[Image:Esophageal adenocarcinoma - intermed mag.jpg|right|thumb|[[Micrograph]] of an esophageal [[adenocarcinoma]] (dark blue - upper-left of image) and normal squamous epithelium (upper-right of image). [[H&E stain]].]] |
||
Esophageal cancers are typically [[carcinoma]]s which arise from the [[epithelium]], or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: [[squamous cell carcinoma]]s, which are similar to [[head and neck cancer]] in their appearance and association with [[tobacco]] and [[alcohol]] consumption, and [[adenocarcinoma]]s, which are often associated with a history of [[gastroesophageal reflux disease]] and [[Barrett's esophagus]]. A general rule of thumb is that a cancer in the upper two-thirds is a squamous cell carcinoma and one in the lower one-third is a adenocarcinoma. Rare histologic |
Esophageal cancers are typically [[carcinoma]]s which arise from the [[epithelium]], or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: [[squamous cell carcinoma]]s, which are similar to [[head and neck cancer]] in their appearance and association with [[tobacco]] and [[alcohol]] consumption, and [[adenocarcinoma]]s, which are often associated with a history of [[gastroesophageal reflux disease]] and [[Barrett's esophagus]]. A general rule of thumb is that a cancer in the upper two-thirds is a squamous cell carcinoma and one in the lower one-third is a adenocarcinoma. Rare histologic types of esophageal cancer are different variants of the squamous cell carcinoma, and non-epithelial tumors, such as [[leiomyosarcoma]], [[malignant melanoma]], [[rhabdomyosarcoma]], [[lymphoma]] and others.<ref>{{cite book |url=http://books.google.com/books?id=bVEEHmpU-1wC&pg=PA2047 |title=Less Common Malignant Tumors of the Esophagus | author=W Shield, Thomas. LoCicero, Joseph. B. Ponn, Ronald. | year=2005 | publisher=Lippincott Williams & Wilkins | pages=2325–2340 | isbn=9780781738897}}</ref><ref name=Perez>{{cite book|title=Perez and Brady's principles and practice of radiation oncology|year=2008|publisher=Lippincott Williams & Wilkins|isbn=9780781763691|pages=1137|url=http://books.google.com/books?id=NyeE6-aKnSYC&pg=PA1137 |author=Halperin, Edward C.|coauthors=Perez, A. Brady, Luther W.}}</ref> |
||
==Signs and symptoms== |
==Signs and symptoms== |
||
[[Dysphagia]] (difficulty swallowing) and [[odynophagia]] (painful swallowing) are the most common symptoms of esophageal cancer. [[Dysphagia]] is the first symptom in most patients. Odynophagia may also be present. Fluids and soft foods are usually tolerated, while hard or bulky substances (such as bread or meat) cause much more difficulty. Substantial weight loss is characteristic as a result of reduced appetite and poor nutrition and the active cancer. Pain behind the [[sternum]] or in the [[epigastrium]], often of a burning, heartburn-like nature, may be severe, present itself almost daily, and is worsened by swallowing any form of food. Another sign may be an unusually husky, raspy, or hoarse sounding cough, a result of the tumor affecting the [[recurrent laryngeal nerve]]. |
[[Dysphagia]] (difficulty swallowing) and [[odynophagia]] (painful swallowing) are the most common symptoms of esophageal cancer. [[Dysphagia]] is the first symptom in most patients. Odynophagia may also be present. Fluids and soft foods are usually tolerated, while hard or bulky substances (such as bread or meat) cause much more difficulty. Substantial weight loss is characteristic as a result of reduced appetite and poor nutrition and the active cancer. Pain behind the [[sternum]] or in the [[epigastrium]], often of a burning, heartburn-like nature, may be severe, present itself almost daily, and is worsened by swallowing any form of food. Another sign may be an unusually husky, raspy, or hoarse sounding cough, a result of the tumor affecting the [[recurrent laryngeal nerve]]. |
||
The presence of the tumor may disrupt normal [[peristalsis]] (the organized swallowing reflex), leading to [[nausea]] and [[vomiting]], [[Regurgitation (digestion)|regurgitation]] of food, [[cough]]ing and an increased risk of [[aspiration pneumonia]]. The tumor surface may be fragile and [[hemorrhage|bleed]], causing [[hematemesis]] (vomiting up blood). Compression of local structures occurs in advanced disease, leading to such problems as [[stridor|upper airway obstruction]] and [[superior vena cava syndrome]]. [[Fistula]]s may develop between the esophagus and the [[Vertebrate trachea|trachea]], increasing the pneumonia risk; this condition is usually heralded by |
The presence of the tumor may disrupt normal [[peristalsis]] (the organized swallowing reflex), leading to [[nausea]] and [[vomiting]], [[Regurgitation (digestion)|regurgitation]] of food, [[cough]]ing and an increased risk of [[aspiration pneumonia]]. The tumor surface may be fragile and [[hemorrhage|bleed]], causing [[hematemesis]] (vomiting up blood). Compression of local structures occurs in advanced disease, leading to such problems as [[stridor|upper airway obstruction]] and [[superior vena cava syndrome]]. [[Fistula]]s may develop between the esophagus and the [[Vertebrate trachea|trachea]], increasing the pneumonia risk; this condition is usually heralded by [[cough]], [[fever]] or aspiration.<ref name=Enzinger/> |
||
Most of the people diagnosed with esophageal cancer have late-stage disease. This is because people usually do not have significant symptoms until half of the inside of the esophagus, called the lumen, is obstructed, by which point the tumor is fairly large. |
Most of the people diagnosed with esophageal cancer have late-stage disease. This is because people usually do not have significant symptoms until half of the inside of the esophagus, called the lumen, is obstructed, by which point the tumor is fairly large. |
||
| Line 53: | Line 53: | ||
* Alcohol consumption in individuals predisposed to [[alcohol flush reaction]]<ref>{{cite journal |author=Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A |title=The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption |journal=PLOS Medicine |volume=6 |issue=3 |pages=191–5 |year=2009 |doi=10.1371/journal.pmed.1000050 |pmid=19320537 |pmc=2659709}}</ref> |
* Alcohol consumption in individuals predisposed to [[alcohol flush reaction]]<ref>{{cite journal |author=Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A |title=The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption |journal=PLOS Medicine |volume=6 |issue=3 |pages=191–5 |year=2009 |doi=10.1371/journal.pmed.1000050 |pmid=19320537 |pmc=2659709}}</ref> |
||
* [[Achalasia]]<ref name=Park_2005>{{cite journal |author=Park W, Vaezi M |title=Etiology and pathogenesis of achalasia: the current understanding |journal=Am J Gastroenterol |volume=100 |issue=6 |pages=1404–14 |year=2005 |pmid=15929777 |doi=10.1111/j.1572-0241.2005.41775.x}}</ref> |
* [[Achalasia]]<ref name=Park_2005>{{cite journal |author=Park W, Vaezi M |title=Etiology and pathogenesis of achalasia: the current understanding |journal=Am J Gastroenterol |volume=100 |issue=6 |pages=1404–14 |year=2005 |pmid=15929777 |doi=10.1111/j.1572-0241.2005.41775.x}}</ref> |
||
*Drugs that relax the valve between the stomach and the esophagus. For example [[anti-cholinergic]] drugs used in Parkinson's Disease and [[beta agonists]] and aminophylline used in asthma. [[http://www.cancerhelp.org.uk/type/oesophageal-cancer/about/risks-and-causes-of-oesophageal-cancer]] |
|||
===Decreased risk=== |
===Decreased risk=== |
||
*Risk appears to be less in patients using [[aspirin]] or related drugs ([[NSAID]]s).<ref>Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. ''Gastroenterology'' 2003;124:47–56. PMID 12512029. See also [http://www.cancer.gov/cancertopics/pdq/prevention/esophageal/healthprofessional#Section_57 NCI - "Esophageal Cancer (PDQ): Prevention"].</ref> |
*Risk appears to be less in patients using [[aspirin]] or related drugs ([[NSAID]]s).<ref>Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. ''Gastroenterology'' 2003;124:47–56. PMID 12512029. See also [http://www.cancer.gov/cancertopics/pdq/prevention/esophageal/healthprofessional#Section_57 NCI - "Esophageal Cancer (PDQ): Prevention"].</ref> |
||
| Line 69: | Line 69: | ||
Although an occlusive tumor may be suspected on a [[barium swallow]] or [[barium meal]], the diagnosis is best made with [[esophagogastroduodenoscopy]] (EGD, [[endoscopy]]); this involves the passing of a flexible tube down the esophagus and visualizing the wall. [[Biopsy|Biopsies]] taken of suspicious lesions are then examined [[histology|histologically]] for signs of malignancy. |
Although an occlusive tumor may be suspected on a [[barium swallow]] or [[barium meal]], the diagnosis is best made with [[esophagogastroduodenoscopy]] (EGD, [[endoscopy]]); this involves the passing of a flexible tube down the esophagus and visualizing the wall. [[Biopsy|Biopsies]] taken of suspicious lesions are then examined [[histology|histologically]] for signs of malignancy. |
||
Additional testing is usually performed to estimate the tumor stage |
Additional testing is usually performed to estimate the tumor stage. [[Computed tomography]] (CT) of the chest, abdomen and pelvis, can evaluate whether the cancer has spread to adjacent tissues or distant organs (especially [[liver]] and [[lymph node]]s). The sensitivity of CT scan is limited by its ability to detect masses (e.g. enlarged lymph nodes or involved organs) generally larger than 1 cm. [[FDG-PET]] (positron emission tomography) scan is also being used to estimate whether enlarged masses are metabolically active, indicating faster-growing cells that might be expected in cancer. Esophageal [[endoscopic ultrasound]] (EUS) can provide staging information regarding the level of tumor invasion, and possible spread to regional lymph nodes. |
||
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 inches long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur distally and squamous cell carcinomas proximally |
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 inches long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur distally and squamous cell carcinomas proximally, but the converse may also be the case. |
||
==Management== |
==Management== |
||
Revision as of 22:06, 4 May 2011
| Esophageal cancer | |
|---|---|
| Specialty | Oncology, general surgery, gastroenterology |
Esophageal cancer (or oesophageal cancer) is malignancy of the esophagus. There are various subtypes, primarily squamous cell cancer (approx 90-95% of all esophageal cancer worldwide) and adenocarcinoma (approx. 50-80% of all esophageal cancer in the United States). Squamous cell cancer arises from the cells that line the upper part of the esophagus. Adenocarcinoma arises from glandular cells that are present at the junction of the esophagus and stomach.[1]
Esophageal tumors usually lead to dysphagia (difficulty swallowing), pain and other symptoms, and are diagnosed with biopsy. Small and localized tumors are treated surgically with curative intent. Larger tumors tend not to be operable and hence are treated with palliative care; their growth can still be delayed with chemotherapy, radiotherapy or a combination of the two. In some cases chemo- and radiotherapy can render these larger tumors operable. Prognosis depends on the extent of the disease and other medical problems, but is fairly poor.[2]
Classification

Esophageal cancers are typically carcinomas which arise from the epithelium, or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: squamous cell carcinomas, which are similar to head and neck cancer in their appearance and association with tobacco and alcohol consumption, and adenocarcinomas, which are often associated with a history of gastroesophageal reflux disease and Barrett's esophagus. A general rule of thumb is that a cancer in the upper two-thirds is a squamous cell carcinoma and one in the lower one-third is a adenocarcinoma. Rare histologic types of esophageal cancer are different variants of the squamous cell carcinoma, and non-epithelial tumors, such as leiomyosarcoma, malignant melanoma, rhabdomyosarcoma, lymphoma and others.[3][4]
Signs and symptoms
Dysphagia (difficulty swallowing) and odynophagia (painful swallowing) are the most common symptoms of esophageal cancer. Dysphagia is the first symptom in most patients. Odynophagia may also be present. Fluids and soft foods are usually tolerated, while hard or bulky substances (such as bread or meat) cause much more difficulty. Substantial weight loss is characteristic as a result of reduced appetite and poor nutrition and the active cancer. Pain behind the sternum or in the epigastrium, often of a burning, heartburn-like nature, may be severe, present itself almost daily, and is worsened by swallowing any form of food. Another sign may be an unusually husky, raspy, or hoarse sounding cough, a result of the tumor affecting the recurrent laryngeal nerve.
The presence of the tumor may disrupt normal peristalsis (the organized swallowing reflex), leading to nausea and vomiting, regurgitation of food, coughing and an increased risk of aspiration pneumonia. The tumor surface may be fragile and bleed, causing hematemesis (vomiting up blood). Compression of local structures occurs in advanced disease, leading to such problems as upper airway obstruction and superior vena cava syndrome. Fistulas may develop between the esophagus and the trachea, increasing the pneumonia risk; this condition is usually heralded by cough, fever or aspiration.[2]
Most of the people diagnosed with esophageal cancer have late-stage disease. This is because people usually do not have significant symptoms until half of the inside of the esophagus, called the lumen, is obstructed, by which point the tumor is fairly large. [5]
If the disease has spread elsewhere, this may lead to symptoms related to this: liver metastasis could cause jaundice and ascites, lung metastasis could cause shortness of breath, pleural effusions, etc.
Causes
Increased risk

There are a number of risk factors for esophageal cancer.[2] Some subtypes of cancer are linked to particular risk factors:
- Age. Most patients are over 60, and the median in US patients is 67.[2]
- Sex. It is more common in men.
- Heredity. It is more likely in people who have close relatives with cancer.
- Tobacco smoking and heavy alcohol use increase the risk, and together appear to increase the risk more than either individually. Tobacco and/or alcohol account for approximately 90% of all esophegeal squamous cell carcinomas. Tobacco smoking is also linked to esophegeal adenocarcinoma though no scientific evidence has been found between alcohol and esophegeal adenocarcinoma.
- Gastroesophageal reflux disease (GERD) and its resultant Barrett's esophagus increase esophageal cancer risk due to the chronic irritation of the mucosal lining. Adenocarcinoma is more common in this condition.[6]
- Human papillomavirus (HPV)[7]
- Corrosive injury to esophagus by swallowing strong alkalines (lye) or acids.
- Particular dietary substances, such as nitrosamine.
- A medical history of other head and neck cancers increases the chance of developing a second cancer in the head and neck area, including esophageal cancer.
- Plummer-Vinson syndrome (anemia and esophageal webbing)
- Tylosis and Howel-Evans syndrome (hereditary thickening of the skin of the palms and soles).
- Radiation therapy for other conditions in the mediastinum.[2]
- Coeliac disease predisposes towards squamous cell carcinoma.[8]
- Obesity increases the risk of adenocarcinoma fourfold.[9] It is suspected that increased risk of reflux may be behind this association.[6][10]
- Thermal injury as a result of drinking hot beverages[11][12]
- Alcohol consumption in individuals predisposed to alcohol flush reaction[13]
- Achalasia[14]
Decreased risk
- Risk appears to be less in patients using aspirin or related drugs (NSAIDs).[15]
- The role of Helicobacter pylori in progression to esophageal adenocarcinoma is still uncertain, but, on the basis of population data, it may carry a protective effect.[16][17] It is postulated that H. pylori prevents chronic gastritis, which is a risk factor for reflux, which in turn is a risk factor for esophageal adenocarcinoma.[18]
- According to the National Cancer Institute, "diets high in cruciferous (cabbage, broccoli, cauliflower) and green and yellow vegetables and fruits are associated with a decreased risk of esophageal cancer."[19]
- Moderate coffee consumption is associated with a decreased risk.[20]
- According to one Italian study of "diet surveys completed by 5,500 Italians"—a study which has raised debates questioning its claims among cancer researchers cited in news reports about it—eating pizza more than once a week appears "to be a favorable indicator of risk for digestive tract neoplasms in this population."[21]
Diagnosis

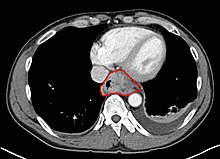
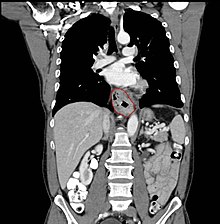
Clinical evaluation
Although an occlusive tumor may be suspected on a barium swallow or barium meal, the diagnosis is best made with esophagogastroduodenoscopy (EGD, endoscopy); this involves the passing of a flexible tube down the esophagus and visualizing the wall. Biopsies taken of suspicious lesions are then examined histologically for signs of malignancy.
Additional testing is usually performed to estimate the tumor stage. Computed tomography (CT) of the chest, abdomen and pelvis, can evaluate whether the cancer has spread to adjacent tissues or distant organs (especially liver and lymph nodes). The sensitivity of CT scan is limited by its ability to detect masses (e.g. enlarged lymph nodes or involved organs) generally larger than 1 cm. FDG-PET (positron emission tomography) scan is also being used to estimate whether enlarged masses are metabolically active, indicating faster-growing cells that might be expected in cancer. Esophageal endoscopic ultrasound (EUS) can provide staging information regarding the level of tumor invasion, and possible spread to regional lymph nodes.
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 inches long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur distally and squamous cell carcinomas proximally, but the converse may also be the case.
Management
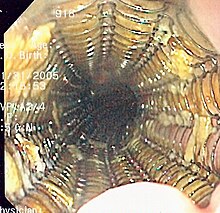
General approaches
The treatment is determined by the cellular type of cancer (adenocarcinoma or squamous cell carcinoma vs other types), the stage of the disease, the general condition of the patient and other diseases present. On the whole, adequate nutrition needs to be assured, and adequate dental care is vital.
If the patient cannot swallow at all, an esophageal stent may be inserted to keep the esophagus patent; stents may also assist in occluding fistulas. A nasogastric tube may be necessary to continue feeding while treatment for the tumor is given, and some patients require a gastrostomy (feeding hole in the skin that gives direct access to the stomach). The latter two are especially important if the patient tends to aspirate food or saliva into the airways, predisposing for aspiration pneumonia.
Esophagectomy is the removal of a segment of the esophagus; as this shortens the length of the remaining esophagus, some other segment of the digestive tract (typically the stomach or part of the Colon or jejunum]) is pulled up to the chest cavity and interposed.[22] If the tumor is unresectable or the patient is not fit for surgery, palliative esophageal stenting can allow the patient to tolerate soft diet.
Types of esophagectomy:
- Thoracoabdominal approach- which opens the abdominal and thoracic cavities together.
- Two stage Ivor Lewis (also called Lewis-Tanner) approach- with an initial laparotomy and construction of a gastric tube, followed by a right thoracotomy to excise the tumor and create an esophagogastric anastomosis.
- Three stage McKeown approach- where a third incision in the neck is made to complete the cervical anastomosis.
Endoscopic Therapy for Localized Disease There is accumulating data that endoscopic therapy is a safe, less invasive, and effective therapy for very early esophageal cancer. The candidates for endoscopic therapy are Stage 1 patients with tumors invading into the lamina propria (T1 mucosal) or submucosa (T1 submucosal) that do not have regional or distant metastasis. Patients with carcinoma in-situ or high-grade dysplasia can also be treated with endoscopic therapy. Submucosa cancers with increased risk of nodal metastases may not be as amenable to curative therapy.
The two forms of endoscopic therapy that have been used for Stage 0 and I disease are endoscopic mucosal resection (EMR) and mucosal ablation using photodynamic therapy , Nd-YAG laser, or argon plasma coagulation.
EMR Endoscopic Mucosal Resection has been advocated for early cancers (that is, those that are superficial and confined to the mucosa only) and has been shown to be a less invasive, safe, and highly effective nonsurgical therapy for early squamous cell esophageal cancer. Preliminary reports also suggest its safety and efficacy for early adenocarcinoma arising in Barrett’s esophagus. The prognosis after treatment with endoscopic mucosal resection is comparable to surgical resection. This technique can be attempted in patients, without evidence of nodal or distant metastases, with differentiated tumors that are slightly raised and less than 2 cm in diameter, or in differentiated tumors that are ulcerated and less than 1 cm in diameter. The most commonly employed modalities of endoscopic mucosal resection include strip biopsy, double-snare polypectomy, resection with combined use of highly concentrated saline and epinephrine, and resection using a cap.
The strip biopsy method for endoscopic mucosal resection of esophageal cancer is performed with a double-channel endoscope equipped with grasping forceps and snare. After marking the lesion border with an electric coagulator, saline is injected into the submucosa below the lesion to separate the lesion from the muscle layer and to force its protrusion. The grasping forceps are passed through the snare loop. The mucosa surrounding the lesion is grasped, lifted, and strangulated and resected by electrocautery. The endoscopic double-snare polypectomy method is indicated for protruding lesions. Using a double-channel scope, the lesion is grasped and lifted by the first snare and strangulated with the second snare for complete resection.
Endoscopic resection with injection of concentrated saline and epinephrine is carried out using a double-channel scope. The lesion borders are marked with a coagulator. Highly concentrated saline and epinephrine are injected (15–20 ml) into the submucosal layer to swell the area containing the lesion and elucidate the markings. The mucosa outside the demarcated border is excised using a high-frequency scalpel to the depth of the submucosal layer. The resected mucosa is lifted and grasped with forceps, trapping and strangulating the lesion with a snare, and then resected by electrocautery.
A fourth method of endoscopic mucosal resection employs the use of a clear cap and prelooped snare inside the cap. After insertion, the cap is placed on the lesion and the mucosa containing the lesion is drawn up inside the cap by aspiration. The mucosa is caught by the snare and strangulated, and finally resected by electrocautery. This is called the "band and snare" or "suck and cut" technique. The resected specimen is retrieved and submitted for microscopic examination for determination of tumor invasion depth, resection margin, and possible vascular involvement. The resulting "ulcer" heals within 3 weeks.
Although most lesions treated in the esophagus have been early squamous cell cancers, endoscopic snare resection can also be used to debulk or completely treat polypoid dysplastic or malignant lesions in Barrett’s esophagus. In a preliminary report from Germany, EMR was performed as primary treatment or adjunctive therapy following photodynamic therapy for early adenocarcinomas in Barrett's esophagus. The "suck and cut" technique (with and without prior saline injection) was used as well as the "band and cut" technique. Although all tumors were resected without difficulty, 12.5% developed bleeding (which was managed successfully by endoscopic therapy). Eighty-one percent of the lesions were completely resected. The other lesions were also treated with other endoscopic techniques. While this report suggests it is feasible to completely resect local, circumscribed, early adenocarcinomas arising in Barrett's esophagus, the relative safety and efficacy of EMR in conjunction with photodynamic therapy is unknown.
The major complications of endoscopic mucosal resection include postoperative bleeding and perforation and stricture formation. During the procedure, an injection of 100,000 times diluted epinephrine into the muscular wall, along with high frequency coagulation or clipping can be applied to the bleeding point for hemostasis. It is important to administer acid-reducing medications to prevent postoperative hemorrhage. Perforation may be prevented with sufficient saline injection to raise the mucosa containing the lesion. The "non-lifting sign" and complaints of pain when the snare strangulates the lesion are contrainidications of EMR. When perforation is recognized immediately after a procedure, the perforation should be closed by clips. Surgery should be considered in cases of endoscopic closure failure. The incidence of complication range from 0–50% and squamous cell recurrence rates range from 0–8%.
Laser therapy is the use of high-intensity light to destroy tumor cells; it affects only the treated area. This is typically done if the cancer cannot be removed by surgery. The relief of a blockage can help to reduce dysphagia and pain. Photodynamic therapy (PDT), a type of laser therapy, involves the use of drugs that are absorbed by cancer cells; when exposed to a special light, the drugs become active and destroy the cancer cells.
Chemotherapy depends on the tumor type, but tends to be cisplatin-based (or carboplatin or oxaliplatin) every three weeks with fluorouracil (5-FU) either continuously or every three weeks. In more recent studies, addition of epirubicin (ECF) was better than other comparable regimens in advanced nonresectable cancer.[23] Chemotherapy may be given after surgery (adjuvant, i.e. to reduce risk of recurrence), before surgery (neoadjuvant) or if surgery is not possible; in this case, cisplatin and 5-FU are used. Ongoing trials compare various combinations of chemotherapy; the phase II/III REAL-2 trial – for example – compares four regimens containing epirubicin and either cisplatin or oxaliplatin and either continuously infused fluorouracil or capecitabine.
Radiotherapy is given before, during or after chemotherapy or surgery, and sometimes on its own to control symptoms. In patients with localised disease but contraindications to surgery, "radical radiotherapy" may be used with curative intent.
Follow-up
Patients are followed up frequently after a treatment regimen has been completed. Frequently, other treatments are necessary to improve symptoms and maximize nutrition.
Prognosis
In general, the prognosis of esophageal cancer is quite poor, because most patients present with advanced disease. By the time the first symptoms such as dysphagia start manifesting themselves, the cancer has already well progressed. The overall five-year survival rate (5YSR) is approximately 15%, with most patients dying within the first year of diagnosis.[24]
Individualized prognosis depends largely on stage. Those with cancer restricted entirely to the esophageal mucosa have about an 80% 5YSR, but submucosal involvement brings this down to less than 50%. Extension into the muscularis propria (muscular layer of the esophageus) has meant a 20% 5YSR and extension to the structures adjacent to the esophagus results in a 7% 5YSR. Patients with distant metastases (who are not candidates for curative surgery) have a less than 3% 5YSR. .
Epidemiology

Esophageal cancer is a relatively rare form of cancer, but some world areas have a markedly higher incidence than others: Belgium, China, Iran, Iceland, India, Japan, the United Kingdom, appear to have a higher incidence, as well as the region around the Caspian Sea.[26] The American Cancer Society estimates that during 2007, approximately 15,560 new esophageal cancer cases will be diagnosed in the United States.[27]
In the United States, squamous cell carcinoma of the esophagus usually affects African-American males with a history of heavy smoking or alcohol use. Up until the 1970s, squamous cell carcinoma made up the vast majority of esophageal cancer in the United States. In recent decades, incidence of adenocarcinoma of the esophagus (which is associated with Barrett's esophagus) steadily rose in the United States to the point that it has now surpassed squamous cell carcinoma in this country. In contrast to squamous cell carcimona, esophageal adenocarcimona is more common in Caucasian men (over the age of 60) than it is in African-Americans. Multiple reports indicate that esophageal adenocarcinoma incidence has increased during the past 20 years, especially in non-Hispanic white men. Esophageal adenocarcinoma age-adjusted incidence increased in New Mexico from 1973 to 1997. This increase was found in non-Hispanic whites and Hispanics and became predominant in non-Hispanic whites.[28] Esophageal cancer incidence and mortality rates for African-Americans continue to be higher than the rate for Causasians. However incidence and mortality of esophageal cancer has significantly decreased among African-Americans since the early 1980s whereas with Caucasians it has slightly increased.[29]
References
- ^ Esophageal cancer at Mount Sinai Hospital
- ^ a b c d e Enzinger PC, Mayer RJ (2003). "Esophageal cancer". N. Engl. J. Med. 349 (23): 2241–52. doi:10.1056/NEJMra035010. PMID 14657432.
- ^ W Shield, Thomas. LoCicero, Joseph. B. Ponn, Ronald. (2005). Less Common Malignant Tumors of the Esophagus. Lippincott Williams & Wilkins. pp. 2325–2340. ISBN 9780781738897.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Halperin, Edward C. (2008). Perez and Brady's principles and practice of radiation oncology. Lippincott Williams & Wilkins. p. 1137. ISBN 9780781763691.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Corrina Wu, "American Eyewitness", CR Magazine, Spring/Summer 2010
- ^ a b Lagergren J, Bergström R, Lindgren A, Nyrén O (1999). "Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma". N. Engl. J. Med. 340 (11): 825–31. doi:10.1056/NEJM199903183401101. PMID 10080844.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Syrjänen KJ (2002). "HPV infections and oesophageal cancer". J. Clin. Pathol. 55 (10): 721–8. doi:10.1136/jcp.55.10.721. PMC 1769774. PMID 12354793. PMC 1769774
- ^ Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI (2003). "Risk of malignancy in patients with celiac disease". Am. J. Med. 115 (3): 191–5. doi:10.1016/S0002-9343(03)00302-4. PMID 12935825.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA (2007). "Body Mass Index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study". Gut. 56 (11): 1503–11. doi:10.1136/gut.2006.116665. PMC 2095659. PMID 17337464.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Layke JC, Lopez PP (2006). "Esophageal cancer: a review and update". American Family Physician. 73 (12): 2187–94. PMID 16836035.
- ^ Islami F, Pourshams A, Nasrollahzadeh D; et al. (2009). "Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study". BMJ. 338: b929. doi:10.1136/bmj.b929. PMID 19325180.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Whiteman DC (2009). "Hot tea and increased risk of oesophageal cancer". BMJ. 338: b610. doi:10.1136/bmj.b610. PMID 19325178.
- ^ Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A (2009). "The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption". PLOS Medicine. 6 (3): 191–5. doi:10.1371/journal.pmed.1000050. PMC 2659709. PMID 19320537.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Park W, Vaezi M (2005). "Etiology and pathogenesis of achalasia: the current understanding". Am J Gastroenterol. 100 (6): 1404–14. doi:10.1111/j.1572-0241.2005.41775.x. PMID 15929777.
- ^ Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56. PMID 12512029. See also NCI - "Esophageal Cancer (PDQ): Prevention".
- ^ Wong A, Fitzgerald RC (2005). "Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma". Clin. Gastroenterol. Hepatol. 3 (1): 1–10. doi:10.1016/S1542-3565(04)00602-0. PMID 15645398.
- ^ Ye W, Held M, Lagergren J; et al. (2004). "Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia". J. Natl. Cancer Inst. 96 (5): 388–96. doi:10.1093/jnci/djh057. PMID 14996860.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Nakajima S, Hattori T (2004). "Oesophageal adenocarcinoma or gastric cancer with or without eradication of Helicobacter pylori infection in chronic atrophic gastritis patients: a hypothetical opinion from a systematic review". Aliment. Pharmacol. Ther. 20 Suppl 1: 54–61. doi:10.1111/j.1365-2036.2004.01975.x. PMID 15298606.
- ^ NCI (2002). "Prevention: Dietary Factors, based on Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer". Nutr Cancer. 44 (2): 104–26. PMID 12734057.
{{cite journal}}: External link in|title= - ^ Tavani, A (2003). "Coffee and tea intake and risk of oral, pharyngeal and esophageal cancer". Oral Oncol. 39 (7): 695–700. doi:10.1016/S1368-8375(03)00081-2. PMID 12907209.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Gallus S, Bosetti C, Negri E, Talamini R, Montella M, Conti E, Franceschi S, La Vecchia C. Does pizza protect against cancer? Int J Cancer 2003;107:283–4. PMID 12949808. Cited and qtd. by WebMD and BBC News.
- ^ Deschamps C, Nichols FC, Cassivi SD; et al. (2005). "Long-term function and quality of life after esophageal resection for cancer and Barrett's". Surgical Clinics of North America. 85 (3): 649–56. doi:10.1016/j.suc.2005.01.018. PMID 15927658.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Ross P, Nicolson M, Cunningham D; et al. (2002). "Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 20 (8): 1996–2004. doi:10.1200/JCO.2002.08.105. PMID 11956258.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Polednak AP (2003). "Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas". Int. J. Cancer. 105 (1): 98–100. doi:10.1002/ijc.11029. PMID 12672037.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved Nov. 11, 2009.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Stewart, Bernard W.; Kleihues, P. (2003). World cancer report. Lyon: IARC Press. ISBN 92-832-0411-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "What Are the Key Statistics About Cancer of the Esophagus?". Detailed Guide: Esophagus Cancer. American Cancer Society. 2006. Archived from the original on 2007-09-29. Retrieved 2007-03-21.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Kenneth J. Vega, M.D., M. Mazen JamaM.D.l (2000). "Changing pattern of esophageal cancer incidence in New Mexico". Changing pattern of esophageal cancer incidence in New Mexico. The American Journal of Gastroenterology. Retrieved 2007-03-21.
{{cite web}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) [dead link] - ^ "Incidence and Mortality Rate Trends" (PDF). A Snapshot of Esophageal Cancer. National Cancer Institute. 2006. Archived from the original (PDF) on 2007-03-16. Retrieved 2007-03-21.
{{cite web}}: Unknown parameter|month=ignored (help)
External links
- NCI Esophageal Cancer Home Page
- Cancer.Net: Esophageal Cancer
- Barrett's Oesophagus Campaign - Working to prevent oesophageal cancer / cancer of the gullet
- MedlinePlus: Esophageal Cancer
- Esophageal Cancer From Cancer Management: A Multidisciplinary Approach
- Learn More about Esophageal Cancer
- Oesophageal Cancer at Cancer Research UK
- Oesophageal Cancer Facts/ Resources (BBC) (Last updated: 30 January 2004)
- Esophageal Cancer Awareness Association Home Page
- Esophageal Cancer Action Network Home Page
