Human tooth development: Difference between revisions
GoingBatty (talk | contribs) m General fixes & manual clean up, replaced: a rare anomaly → an anomaly, replaced: .After → . After using AWB (10482) |
m RuneMan3 moved page Tooth development to Human tooth development: I am about to split this page. |
(No difference)
| |
Revision as of 04:14, 19 December 2014
This article may be too technical for most readers to understand. (September 2010) |

Tooth development or odontogenesis is the complex process by which teeth form from embryonic cells, grow, and erupt into the mouth. Although many diverse species have teeth, non-human tooth development is largely the same as in humans. For human teeth to have a healthy oral environment, enamel, dentin, cementum, and the periodontium must all develop during appropriate stages of fetal development. Primary (baby) teeth start to form between the sixth and eighth week of prenatal development, and permanent teeth begin to form in the twentieth week.[1] If teeth do not start to develop at or near these times, they will not develop at all, resulting in hypodontia or anodontia.
A significant amount of research has focused on determining the processes that initiate tooth development. It is widely accepted that there is a factor within the tissues of the first branchial arch that is necessary for the development of teeth.[1]
In vertebrates, several specializations of epithelial tissue ('phanères') generate after thickening specific structures: keratinized structure (hair, nails) or exoskeletons structure (scales, teeth). Placoids scales and teeth of sharks are considered homologous organs.[2]
Overview
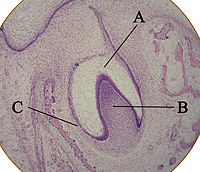
A: enamel organ
B: dental papilla
C: dental follicle
The tooth germ is an aggregation of cells that eventually forms a tooth.[3] These cells are derived from the ectoderm of the first branchial arch and the ectomesenchyme of the neural crest.[1][4][5] The tooth germ is organized into three parts: the enamel organ, the dental papilla and the dental sac or follicle.
The enamel organ is composed of the outer enamel epithelium, inner enamel epithelium, stellate reticulum and stratum intermedium.[3] These cells give rise to ameloblasts, which produce enamel and become a part of the reduced enamel epithelium (REE) after maturation of the enamel. The location where the outer enamel epithelium and inner enamel epithelium join is called the cervical loop.[1] The growth of cervical loop cells into the deeper tissues forms Hertwig Epithelial Root Sheath, which determines the root shape of the tooth.[2]
The dental papilla contains cells that develop into odontoblasts, which are dentin-forming cells.[3] Additionally, the junction between the dental papilla and inner enamel epithelium determines the crown shape of a tooth.[1] Mesenchymal cells within the dental papilla are responsible for formation of tooth pulp.
The dental sac or follicle gives rise to three important entities: cementoblasts, osteoblasts, and fibroblasts. Cementoblasts form the cementum of a tooth. Osteoblasts give rise to the alveolar bone around the roots of teeth. Fibroblasts are involved developing the periodontal ligament which connect teeth to the alveolar bone through cementum.[6]
Human tooth development timeline
The following tables present the development timeline of human teeth.[7] Times for the initial calcification of primary teeth are for weeks in utero. Abbreviations: wk = weeks; mo = months; yr = years.
| Maxillary (upper) teeth | ||||||||
|---|---|---|---|---|---|---|---|---|
| Primary teeth | Central incisor |
Lateral incisor |
Canine |
First molar |
Second molar | |||
| Initial calcification | 14 wk I.U. | 16 wk I.U. | 17 wk I.U. | 15.5 wk I.U. | 19 wk I.U. | |||
| Crown completed | 1.5 mo | 2.5 mo | 9 mo | 6 mo | 11 mo | |||
| Root completed | 1.5 yr | 2 yr | 3.25 yr | 2.5 yr | 3 yr | |||
| Mandibular (lower) teeth | ||||||||
| Initial calcification | 14 wk I.U. | 16 wk I.U. | 17 wk I.U. | 15.5 wk I.U. | 18 wk I.U. | |||
| Crown completed | 2.5 mo | 3 mo | 9 mo | 5.5 mo | 10 mo | |||
| Root completed | 1.5 yr | 1.5 yr | 3.25 yr | 2.5 yr | 3 yr | |||
| Maxillary (upper) teeth | ||||||||
|---|---|---|---|---|---|---|---|---|
| Permanent teeth | Central incisor |
Lateral incisor |
Canine |
First premolar |
Second premolar |
First molar |
Second molar |
Third molar |
| Initial calcification | 3–4 mo | 10–12 mo | 4–5 mo | 1.5–1.75 yr | 2–2.25 yr | at birth | 2.5–3 yr | 7–9 yr |
| Crown completed | 4–5 yr | 4–5 yr | 6–7 yr | 5–6 yr | 6–7 yr | 2.5–3 yr | 7–8 yr | 12–16 yr |
| Root completed | 10 yr | 11 yr | 13–15 yr | 12–13 yr | 12–14 yr | 9–10 yr | 14–16 yr | 18–25 yr |
| Mandibular (lower) teeth | ||||||||
| Initial calcification | 3–4 mo | 3–4 mo | 4–5 mo | 1.5–2 yr | 2.25–2.5 yr | at birth | 2.5–3 yr | 8–10 yr |
| Crown completed | 4–5 yr | 4–5 yr | 6–7 yr | 5–6 yr | 6–7 yr | 2.5–3 yr | 7–8 yr | 12–16 yr |
| Root completed | 9 yr | 10 yr | 12–14 yr | 12–13 yr | 13–14 yr | 9–10 yr | 14–15 yr | 18–25 yr |
Stages

Tooth development is commonly divided into the following stages: the initiation stage, the bud stage, the cap stage, the bell stage, and finally maturation. The staging of tooth development is an attempt to categorize changes that take place along a continuum; frequently it is difficult to decide what stage should be assigned to a particular developing tooth. This determination is further complicated by the varying appearance of different histologic sections of the same developing tooth, which can appear to be different stages.[1]
Initiation Stage
One of the earliest signs in the formation of a tooth that can be seen microscopically is the distinction between the vestibular lamina and the dental lamina. The dental lamina connects the developing tooth bud to the epithelial layer of the mouth for a significant time.[8] This is regarded as the initiation stage.[1]
Bud stage
The bud stage is characterized by the appearance of a tooth bud without a clear arrangement of cells. The stage technically begins once epithelial cells proliferate into the ectomesenchyme of the jaw.[1] Typically, this occurs when the fetus is around 8 weeks old.[9] The tooth bud itself is the group of cells at the periphery of the dental lamina.
Along with the formation of the dental lamina, 10 round epithelial structures, each referred to as a bud, develop at the distal aspect of the dental lamina of each arch. These correspond to the 10 primary teeth of each dental arch, and they signify the bud stage of tooth development. Each bud is separated from the ectomesenchyme by a basement membrane. Ectomesenchymal cells congregate deep to the bud, forming a cluster of cells, which is the initiation of the condensation of the ectomesenchyme. The remaining ectomesenchymal cells are arranged in a more or less haphazardly uniform fashion.
Cap stage

The first signs of an arrangement of cells in the tooth bud occur in the cap stage. A small group of ectomesenchymal cells stops producing extracellular substances, which results in an aggregation of these cells called the dental papilla. At this point, the tooth bud grows around the ectomesenchymal aggregation, taking on the appearance of a cap, and becomes the enamel (or dental) organ covering the dental papilla. A condensation of ectomesenchymal cells called the dental sac or follicle surrounds the enamel organ and limits the dental papilla. Eventually, the enamel organ will produce enamel, the dental papilla will produce dentin and pulp, and the dental sac will produce all the supporting structures of a tooth, the periodontium.[1]

Bell stage
The bell stage is known for the histodifferentiation and morphodifferentiation that takes place. The dental organ is bell-shaped during this stage, and the majority of its cells are called stellate reticulum because of their star-shaped appearance. The bell stage is divided into the early bell stage and the late bell stage.[1] Cells on the periphery of the enamel organ separate into four important layers. Cuboidal cells on the periphery of the dental organ are known as outer enamel epithelium (OEE).[3] The columnar cells of the enamel organ adjacent to the enamel papilla are known as inner enamel epithelium (IEE). The cells between the IEE and the stellate reticulum form a layer known as the stratum intermedium. The rim of the enamel organ where the outer and inner enamel epithelium join is called the cervical loop.[10] In summary, the layers in order of innermost to outermost consist of dentin, enamel (formed by IEE, or 'ameloblasts', as they move outwards/upwards), inner enamel epithelium and stratum intermedium (stratified cells that support the synthetic activity of the inner enamel epithelium) What follows is part of the initial 'enamel organ', the center of which is made up of stellate reticulum cells that serve to protect the enamel organ. This is all encased by the OEE layer.
Other events occur during the bell stage. The dental lamina disintegrates, leaving the developing teeth completely separated from the epithelium of the oral cavity; the two will not join again until the final eruption of the tooth into the mouth.[1]

The crown of the tooth, which is influenced by the shape of the inner enamel epithelium, also takes shape during this stage. Throughout the mouth, all teeth undergo this same process; it is still uncertain why teeth form various crown shapes—for instance, incisors versus canines. There are two dominant hypotheses. The "field model" proposes there are components for each type of tooth shape found in the ectomesenchyme during tooth development. The components for particular types of teeth, such as incisors, are localized in one area and dissipate rapidly in different parts of the mouth. Thus, for example, the "incisor field" has factors that develop teeth into incisor shape, and this field is concentrated in the central incisor area, but decreases rapidly in the canine area. The other dominant hypothesis, the "clone model", proposes that the epithelium programs a group of ectomesenchymal cells to generate teeth of particular shapes. This group of cells, called a clone, coaxes the dental lamina into tooth development, causing a tooth bud to form. Growth of the dental lamina continues in an area called the "progress zone". Once the progress zone travels a certain distance from the first tooth bud, a second tooth bud will start to develop. These two models are not necessarily mutually exclusive, nor does widely accepted dental science consider them to be so: it is postulated that both models influence tooth development at different times.[1]
Other structures that may appear in a developing tooth in this stage are enamel knots, enamel cords, and enamel niche.[1]
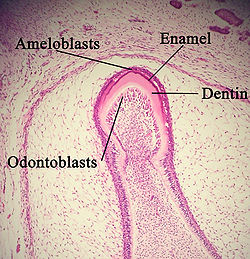
Advanced bell stage
Hard tissues, including enamel and dentin, develop during the next stage of tooth development. This stage is called the crown, or maturation, stage by some researchers. Important cellular changes occur at this time. In prior stages, all of the IEE cells were dividing to increase the overall size of the tooth bud, but rapid dividing, called mitosis, stops during the crown stage at the location where the cusps of the teeth form. The first mineralized hard tissues form at this location. At the same time, the IEE cells change in shape from cuboidal to columnar and become preameloblasts. The nuclei of these cells move closer to the stratum intermedium and away from the dental papilla as they become polarized.[1]

A: enamel
B: dentin
The adjacent layer of cells in the dental papilla suddenly increases in size and differentiates into odontoblasts, which are the cells that form dentin.[11] Researchers believe that the odontoblasts would not form if it were not for the changes occurring in the IEE. As the changes to the IEE and the formation of odontoblasts continue from the tips of the cusps, the odontoblasts secrete a substance, an organic matrix, into their immediate surrounding. The organic matrix contains the material needed for dentin formation. As odontoblasts deposit organic matrix termed predentin, they migrate toward the center of the dental papilla. Thus, unlike enamel, dentin starts forming in the surface closest to the outside of the tooth and proceeds inward. Cytoplasmic extensions are left behind as the odontoblasts move inward. The unique, tubular microscopic appearance of dentin is a result of the formation of dentin around these extensions.[1]
After dentin formation begins, the cells of the IEE secrete an organic matrix against the dentin. This matrix immediately mineralizes and becomes the initial layer of the tooth's enamel. Outside the dentin are the newly formed ameloblasts in response to the formation of dentin, which are cells that continue the process of enamel formation; therefore, enamel formation moves outwards, adding new material to the outer surface of the developing tooth.
Hard tissue formation
Enamel
Enamel formation is called amelogenesis and occurs in the crown stage (advanced bell stage) of tooth development. "Reciprocal induction" governs the relationship between the formation of dentin and enamel; dentin formation must always occur before enamel formation.[12] Generally, enamel formation occurs in two stages: the secretory and maturation stages.[13] Proteins and an organic matrix form a partially mineralized enamel in the secretory stage; the maturation stage completes enamel mineralization.
In the secretory stage, ameloblasts release enamel proteins that contribute to the enamel matrix, which is then partially mineralized by the enzyme alkaline phosphatase.[14] This mineralized phase occurs very early around the 3rd or 4th month of pregnancy. This marks the first appearance of enamel in the body. Ameloblasts make enamel at the location of where the cusps of the teeth are located. Enamel grows outwards, away from the center of the tooth. The appearance of this mineralized tissue, which occurs usually around the third or fourth month of pregnancy, marks the first appearance of enamel in the body. Ameloblasts deposit enamel at the location of what become cusps of teeth alongside dentin. Enamel formation then continues outward, away from the center of the tooth.
In the maturation stage, the ameloblasts transport some of the substances used in enamel formation out of the enamel. Thus, the function of ameloblasts changes from enamel production, as occurs in the secretory stage, to transportation of substances. Most of the materials transported by ameloblasts in this stage are proteins used to complete mineralization. The important proteins involved are amelogenins, ameloblastins, enamelins, and tuftelins.[15] By the end of this stage, the enamel has completed its mineralization.
A residue may form on newly erupted teeth of both dentitions that may leave the teeth extrinsically stained. This green-gray residue, Nasmyth membrane, consists of the fused tissue of the reduced enamel epithelium and oral epithelium, as well as the dental cuticle placed by the ameloblasts on the newly formed outer enamel surface. Nasmyth membrane then easily picks up stain from food debris and is hard to remove except by selective polishing. After teeth are erupted there is some residue on the new teeth called Nasmyth membrane. It is a greenish-grey layer that can leave teeth stained. It consists of tissue and oral epithelium and can easily pick up stain from food and liquid particles and can only be removed by selective polishing. The child’s supervising adults may need reassurance that it is only an extrinsic stain on a child’s newly erupted teeth.[16]
Dentin
Dentin formation, known as dentinogenesis, is the first identifiable feature in the crown stage of tooth development. The formation of dentin must always occur before the formation of enamel. The different stages of dentin formation result in different types of dentin: mantle dentin, primary dentin, secondary dentin, and tertiary dentin.
Odontoblasts, the dentin-forming cells, differentiate from cells of the dental papilla. They begin secreting an organic matrix around the area directly adjacent to the inner enamel epithelium, closest to the area of the future cusp of a tooth. The organic matrix contains collagen fibers with large diameters (0.1–0.2 μm in diameter).[17] The odontoblasts begin to move toward the center of the tooth, forming an extension called the odontoblast process.[1] Thus, dentin formation proceeds toward the inside of the tooth. The odontoblast process causes the secretion of hydroxyapatite crystals and mineralization of the matrix. This area of mineralization is known as mantle dentin and is a layer usually about 150 μm thick.[17]
Whereas mantle dentin forms from the preexisting ground substance of the dental papilla, primary dentin forms through a different process. Odontoblasts increase in size, eliminating the availability of any extracellular resources to contribute to an organic matrix for mineralization. Additionally, the larger odontoblasts cause collagen to be secreted in smaller amounts, which results in more tightly arranged, heterogeneous nucleation that is used for mineralization. Other materials (such as lipids, phosphoproteins, and phospholipids) are also secreted.[17]
Secondary dentin is formed after root formation is finished and occurs at a much slower rate. It is not formed at a uniform rate along the tooth, but instead forms faster along sections closer to the crown of a tooth.[18] This development continues throughout life and accounts for the smaller areas of pulp found in older individuals.[17] Tertiary dentin, also known as reparative dentin, forms in reaction to stimuli, such as attrition or dental caries.[19]

A: dentin
B: cementum
Cementum
Cementum formation is called cementogenesis and occurs late in the development of teeth. Cementoblasts are the cells responsible for cementogenesis. Two types of cementum form: cellular and acellular.[20]
Acellular cementum forms first. The cementoblasts differentiate from follicular cells, which can only reach the surface of the tooth's root once Hertwig's Epithelial Root Sheath (HERS) has begun to deteriorate. The cementoblasts secrete fine collagen fibrils along the root surface at right angles before migrating away from the tooth. As the cementoblasts move, more collagen is deposited to lengthen and thicken the bundles of fibers. Noncollagenous proteins, such as bone sialoprotein and osteocalcin, are also secreted.[21] Acellular cementum contains a secreted matrix of proteins and fibers. As mineralization takes place, the cementoblasts move away from the cementum, and the fibers left along the surface eventually join the forming periodontal ligaments.
Cellular cementum develops after most of the tooth formation is complete and after the tooth occludes (in contact) with a tooth in the opposite arch.[21] This type of cementum forms around the fiber bundles of the periodontal ligaments. The cementoblasts forming cellular cementum become trapped in the cementum they produce.
The origin of the formative cementoblasts is believed to be different for cellular cementum and acellular cementum. One of the major current hypotheses is that cells producing cellular cementum migrate from the adjacent area of bone, while cells producing acellular cementum arise from the dental follicle.[21] Nonetheless, it is known that cellular cementum is usually not found in teeth with one root.[21] In premolars and molars, cellular cementum is found only in the part of the root closest to the apex and in interradicular areas between multiple roots.

A: tooth
B: gingiva
C: bone
D: periodontal ligaments
Formation of the periodontium
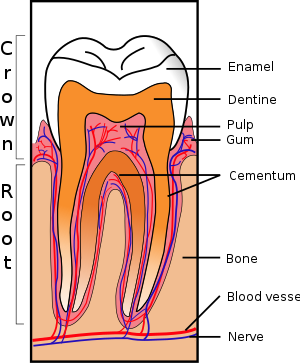
The periodontium, which is the supporting structure of a tooth, consists of the cementum, periodontal ligaments, gingiva, and alveolar bone. Cementum is the only one of these that is a part of a tooth. Alveolar bone surrounds the roots of teeth to provide support and creates what is commonly called a "socket". Periodontal ligaments connect the alveolar bone to the cementum, and the gingiva is the surrounding tissue visible in the mouth.[22]
Periodontal ligament
Cells from the dental follicle give rise to the periodontal ligament (PDL). Specific events leading to the formation of the periodontal ligament vary between deciduous (baby) and permanent teeth and among various species of animals.[21] Nonetheless, formation of the periodontal ligament begins with ligament fibroblasts from the dental follicle. These fibroblasts secrete collagen, which interacts with fibers on the surfaces of adjacent bone and cementum.[23] This interaction leads to an attachment that develops as the tooth erupts into the mouth. The occlusion, which is the arrangement of teeth and how teeth in opposite arches come in contact with one another, continually affects the formation of periodontal ligament. This perpetual creation of periodontal ligament leads to the formation of groups of fibers in different orientations, such as horizontal and oblique fibers.[21]
Alveolar bone
As root and cementum formation begin, bone is created in the adjacent area. Throughout the body, cells that form bone are called osteoblasts. In the case of alveolar bone, these osteoblast cells form from the dental follicle.[21] Similar to the formation of primary cementum, collagen fibers are created on the surface nearest the tooth, and they remain there until attaching to periodontal ligaments.
Like any other bone in the human body, alveolar bone is modified throughout life. Osteoblasts create bone and osteoclasts destroy it, especially if force is placed on a tooth.[24] As is the case when movement of teeth is attempted through orthodontics using bands, wires, or appliances, an area of bone under compressive force from a tooth moving toward it has a high osteoclast level, resulting in bone resorption. An area of bone receiving tension from periodontal ligaments attached to a tooth moving away from it has a high number of osteoblasts, resulting in bone formation. Thus, the tooth or teeth are slowly moved along the jaw so as to achieve a dentition that works in harmony. In this way, the width of the space between the alveoli and the root is kept about the same.[16]
Gingiva
The connection between the gingiva and the tooth is called the dentogingival junction. This junction has three epithelial types: gingival, sulcular, and junctional epithelium. These three types form from a mass of epithelial cells known as the epithelial cuff between the tooth and the mouth.[21]
Much about gingival formation is not fully understood, but it is known that hemidesmosomes form between the gingival epithelium and the tooth and are responsible for the primary epithelial attachment.[21] Hemidesmosomes provide anchorage between cells through small filament-like structures provided by the remnants of ameloblasts. Once this occurs, junctional epithelium forms from reduced enamel epithelium, one of the products of the enamel organ, and divides rapidly. This results in the perpetually increasing size of the junctional epithelial layer and the isolation of the remnants of ameloblasts from any source of nutrition. As the ameloblasts degenerate, a gingival sulcus is created.
Nerve and vascular formation
Frequently, nerves and blood vessels run parallel to each other in the body, and the formation of both usually takes place simultaneously and in a similar fashion. However, this is not the case for nerves and blood vessels around the tooth, because of different rates of development.[1]
Nerve formation
Nerve fibers start to near the tooth during the cap stage of tooth development and grow toward the dental follicle. Once there, the nerves develop around the tooth bud and enter the dental papilla when dentin formation has begun. Nerves never proliferate into the enamel organ.[1]
Vascular formation
Blood vessels grow in the dental follicle and enter the dental papilla in the cap stage.[1] Groups of blood vessels form at the entrance of the dental papilla. The number of blood vessels reaches a maximum at the beginning of the crown stage, and the dental papilla eventually forms in the pulp of a tooth. Throughout life, the amount of pulpal tissue in a tooth decreases, which means that the blood supply to the tooth decreases with age.[24] The enamel organ is devoid of blood vessels because of its epithelial origin, and the mineralized tissues of enamel and dentin do not need nutrients from the blood.
Tooth eruption
Tooth eruption occurs when the teeth enter the mouth and become visible. Although researchers agree that tooth eruption is a complex process, there is little agreement on the identity of the mechanism that controls eruption.[25] Some commonly held theories that have been disproven over time include: (1) the tooth is pushed upward into the mouth by the growth of the tooth's root, (2) the tooth is pushed upward by the growth of the bone around the tooth, (3) the tooth is pushed upward by vascular pressure, and (4) the tooth is pushed upward by the cushioned hammock.[26] The cushioned hammock theory, first proposed by Harry Sicher, was taught widely from the 1930s to the 1950s. This theory postulated that a ligament below a tooth, which Sicher observed under a microscope on a histologic slide, was responsible for eruption. Later, the "ligament" Sicher observed was determined to be merely an artifact created in the process of preparing the slide.[27]
The most widely held current theory is that while several forces might be involved in eruption, the periodontal ligaments provide the main impetus for the process. Theorists hypothesize that the periodontal ligaments promote eruption through the shrinking and cross-linking of their collagen fibers and the contraction of their fibroblasts.[28]
Although tooth eruption occurs at different times for different people, a general eruption timeline exists. Typically, humans have 20 primary (baby) teeth and 32 permanent teeth.[29] Tooth eruption has three stages. The first, known as deciduous dentition stage, occurs when only primary teeth are visible. Once the first permanent tooth erupts into the mouth, the teeth are in the mixed (or transitional) dentition. After the last primary tooth falls out of the mouth—a process known as exfoliation—the teeth are in the permanent dentition.
Primary dentition starts on the arrival of the mandibular central incisors, usually at eight months, and lasts until the first permanent molars appear in the mouth, usually at six years.[30] The primary teeth typically erupt in the following order: (1) central incisor, (2) lateral incisor, (3) first molar, (4) canine, and (5) second molar.[31] As a general rule, four teeth erupt for every six months of life, mandibular teeth erupt before maxillary teeth, and teeth erupt sooner in females than males.[32] During primary dentition, the tooth buds of permanent teeth develop below the primary teeth, close to the palate or tongue.
Mixed dentition starts when the first permanent molar appears in the mouth, usually at six years, and lasts until the last primary tooth is lost, usually at eleven or twelve years.[33] Permanent teeth in the maxilla erupt in a different order from permanent teeth on the mandible. Maxillary teeth erupt in the following order: (1) first molar (2) central incisor, (3) lateral incisor, (4) first premolar, (5) second premolar, (6) canine, (7) second molar, and (8) third molar. Mandibular teeth erupt in the following order: (1) first molar (2) central incisor, (3) lateral incisor, (4) canine, (5) first premolar, (6) second premolar, (7) second molar, and (8) third molar. Since there are no premolars in the primary dentition, the primary molars are replaced by permanent premolars.[34] If any primary teeth are lost before permanent teeth are ready to replace them, some posterior teeth may drift forward and cause space to be lost in the mouth.[35] This may cause crowding and/or misplacement once the permanent teeth erupt, which is usually referred to as malocclusion. Orthodontics may be required in such circumstances for an individual to achieve a straight set of teeth.
The permanent dentition begins when the last primary tooth is lost, usually at 11 to 12 years, and lasts for the rest of a person's life or until all of the teeth are lost (edentulism). During this stage, third molars (also called "wisdom teeth") are frequently extracted because of decay, pain or impactions. The main reasons for tooth loss are decay and periodontal disease.[36]
| Primary teeth | ||||||||
|---|---|---|---|---|---|---|---|---|
| Central incisor |
Lateral incisor |
Canine |
First premolar |
Second premolar |
First molar |
Second molar |
Third molar | |
| Maxillary teeth | 10 mo | 11 mo | 19 mo | 16 mo | 29 mo | |||
| Mandibular teeth | 8 mo | 13 mo | 20 mo | 16 mo | 27 mo | |||
| Permanent teeth | ||||||||
| Central incisor |
Lateral incisor |
Canine |
First premolar |
Second premolar |
First molar |
Second molar |
Third molar | |
| Maxillary teeth | 7–8 yr | 8–9 yr | 11–12 yr | 10–11 yr | 10–12 yr | 6–7 yr | 12–13 yr | 17–21 yr |
| Mandibular teeth | 6–7 yr | 7–8 yr | 9–10 yr | 10–12 yr | 11–12 yr | 6–7 yr | 11–13 yr | 17–21 yr |
Immediately after the eruption enamel is covered by a specific film: Nasmyth's membrane or 'enamel cuticle', structure of embryological origin is composed of keratin which gives rise to the enamel organ.[38][39]
Nutrition and tooth development
As in other aspects of human growth and development, nutrition has an effect on the developing tooth. Essential nutrients for a healthy tooth include calcium, phosphorus, and vitamins A, C, and D.[40] Calcium and phosphorus are needed to properly form the hydroxyapatite crystals, and their levels in the blood are maintained by Vitamin D. Vitamin A is necessary for the formation of keratin, as Vitamin C is for collagen. Fluoride is incorporated into the hydroxyapatite crystal of a developing tooth and makes it more resistant to demineralization and subsequent decay.[23]
Deficiencies of these nutrients can have a wide range of effects on tooth development.[41] In situations where calcium, phosphorus, and vitamin D are deficient, the hard structures of a tooth may be less mineralized. A lack of vitamin A can cause a reduction in the amount of enamel formation. Fluoride deficiency causes increased demineralization when the tooth is exposed to an acidic environment, and also delays remineralization. Furthermore, an excess of fluoride while a tooth is in development can lead to a condition known as fluorosis.
Celiac disease can cause dental enamel defects in children.[42] Bisphenol A (BPA) is a hormone-disrupting chemical that has been implicated in having negative effects on human health, including, but not limited to, fetal development. As shown in animal studies which mimic human enamel, the mother’s consumption of products with BPA during pregnancy can lead to the child’s tooth development being obstructed. Those children are shown to be prone to incisor and first molar hypomineralization, a weakened state of the enamel. Additionally, it is most important for mother’s to avoid BPA during pregnancy, but also avoid BPA-use in the child’s products up to five months of age.[99]
Though reason is not fully understood, high levels of cheese consumption during pregnancy may reduce the risk of childhood caries. Reducing childhood caries leads to a higher quality of life by reducing pain and discomfort.[100]
Developmental disturbances
Anodontia is a complete lack of tooth development, and hypodontia is a lack of some tooth development. Anodontia is rare, most often occurring in a condition called Hypohidrotic ectodermal dysplasia, while hypodontia is one of the most common developmental abnormalities, affecting 3.5–8.0% of the population (not including third molars). The absence of third molars is very common, occurring in 20–23% of the population, followed in prevalence by the second premolar and lateral incisor. Hypodontia is often associated with the absence of a dental lamina, which is vulnerable to environmental forces, such as infection and chemotherapy medications, and is also associated with many syndromes, such as Down syndrome and Crouzon syndrome.[43]
Hyperdontia is the development of extraneous teeth. It occurs in 1–3% of Caucasians and is more frequent in Asians.[44] About 86% of these cases involve a single extra tooth in the mouth, most commonly found in the maxilla, where the incisors are located.[45] Hyperdontia is believed to be associated with an excess of dental lamina.
Dilaceration is an abnormal bend found on a tooth, and is nearly always associated with trauma that moves the developing tooth bud. As a tooth is forming, a force can move the tooth from its original position, leaving the rest of the tooth to form at an abnormal angle. Cysts or tumors adjacent to a tooth bud are forces known to cause dilaceration, as are primary (baby) teeth pushed upward by trauma into the gingiva where it moves the tooth bud of the permanent tooth.[46]
Regional odontodysplasia is rare, but is most likely to occur in the maxilla and anterior teeth. The cause is unknown; a number of causes have been postulated, including a disturbance in the neural crest cells, infection, radiation therapy, and a decrease in vascular supply (the most widely held hypothesis).[47] Teeth affected by regional odontodysplasia nevAmelogenesis imperfecta is an autosomal dominant disease characterized by a defect in dental enamel formation. Teeth are often free of enamel, small, misshapen, and tinted brown. The cause of these deformities is due to a mutation in enamel in expression. Dental patients with this disease should be especially cautious and visit their dentist frequently.[98] Natal and neonatal teeth are an anomaly that involves teeth erupting in a newborn infant’s mouth earlier than usual. Natal teeth are present at the time of birth, occurring in 1:1000 births. Neonatal teeth will erupt during the first 30 days after birth, occurring in 1:30,000 births.[97] Although both conditions occur more frequently in females, natal teeth are three times more common than neonatal teeth. The most common location being the mandibular region of the central incisors; natal and neonatal cuspids are extremely rare.[97] Natal teeth and neonatal teeth are associated with genetics, developmental abnormalities and certain recognized syndromes. Additional names for this condition include precocious dentition, baby teeth, and milk teeth.
Molecular biology
In fish hox gene expression regulates mechanisms for teeth initiation.[48][49]
In mouse WNT signals are required for the initiation of teeth development.[50][51]
NGF-R was present in the condensing ecto-mesenchymal cells of the dental papilla in the early cap stage tooth germ [52] and play multiple roles during morphogenetic and cytodifferentiation events in the tooth.[53][54][55] There is a relationship between tooth agenesis and absence of the peripheral trigeminal nerve (see Hypodontia).
All stages (bud, cap, bell, crown), growth and morphogenesis of the teeth are regulated by a protein: sonic hedgehog.[50][56][57][58]
During tooth development there are strong similarities between keratinization and amelogenesis.[59][60] Keratin is also present in epithelial cells of tooth germ [61] and a thin film of keratin is present on the tooth erupted recently (Nasmyth's membrane or enamel cuticle).[62]
Enamel knots as a signaling center in the tooth morphogenesis and odontoblast differentiation.[63][64][65][66]
Various phenotypic inputs modulate the size of the teeth.[67]
The shape of the teeth in prehistoric man was different from that of modern man.[67][68]
In some dermoid teratomas (particularly ovarian, lung, pancreas, testes) develop complete teeth.[69][70][71][72]
For the tooth eruption is necessary parathyroid hormone.[73]
Hypoparathyroidism patients may suffer from delayed tooth development or even lack of development leading to absence of the teeth, short rounded tooth roots, and malformed/missing enamel. Parathyroid hormone allows calcium to remain in the blood through actions that prevent it from being emptied into the urine, so with low or absent levels of this hormone, the patient may have too much calcium in the urine, kidney stones, or even kidney damage.[102]
Tooth development in animals
Invertebrate "teeth"
True teeth are unique to vertebrates,[74] although some invertebrates have structures have analogous structures sometimes called "teeth" - the organism with the simplest genome bearing such "teeth" is probably the worm genus Ancylostoma (Ancylostoma duodenale, Necator americanus).[75] Molluscs have a structure called a radula which bears a ribbon of chitinous "teeth". However, these are histologically and developmentally different from vertebrate teeth, and are unlikely to be homologous. For example, vertebrate teeth develop from a neural crest mesenchyme-derived dental papilla, and the neural crest is specific to vertebrates, as are tissues such as enamel.[74]
Tooth development in vertebrates
Teeth is atavic structure and their development is similar in many vertebrates.[57][76][77][78][79][80]
Fish have many specialized bony structures,[81] it exist with (Archosargus probatocephalus order Perciformes, family Sparidae) and without teeth (Caristiidae order Perciformes, family Caristiidae, teeth in traces present in juveniles).[82]
Unlike most animals, sharks continuously produce new teeth throughout life[83][84][85] via a drastically different mechanism. Because shark teeth have no roots, sharks easily lose teeth when they feed (zoologists estimate that a single shark can lose up to 2,400 teeth in one year[86])—they must therefore be continually replaced. Shark teeth form from modified scales near the tongue and move outward on the jaw in rows until they fully develop, are used, and are eventually dislodged.[87]
Snakes generally have teeth, with some exception (African Egg-eating Snake).
Today, birds do not have teeth, though it is speculated that prehistoric birds, such as archaeopteryx, did.[88]
In order Tubulidentata (Class Mammalia) teeth are without enamel, they lack incisors and canines and the molars are growing continuously from the root.[89]
Generally, tooth development in non-human mammals is similar to human tooth development. The variations lie in the morphology, number, development timeline, and types of teeth, not usually in the actual development of the teeth.
Enamel formation in non-human mammals is almost identical to that in humans. The ameloblasts and enamel organ, including the dental papilla, function similarly.[90] Nonetheless, while ameloblasts die in humans and most other animals—making further enamel formation impossible—rodents continually produce enamel, forcing them to wear down their teeth by gnawing on various materials.[91] If rodents are prevented from gnawing, their teeth eventually puncture the roofs of their mouths. In addition, rodent incisors consist of two halves, known as the crown and root analogues. The labial half is covered with enamel and resembles a crown, while the lingual half is covered with dentin and resembles a root. Both root and crown develop simultaneously in the rodent incisor and continue to grow for the life of the rodent.
The mineral distribution in rodent enamel is different from that of monkeys, dogs, pigs, and humans.[92] In horse teeth, the enamel and dentin layers are intertwined, which increases the strength and decreases the wear rate of the teeth.[93][94]
Supporting structures that create a "socket" are found exclusively in Mammalia and Crocodylia.[21] In manatees, mandibular molars develop separately from the jaw, and are encased in a bony shell separated by soft tissue. This also occurs in elephants' successional teeth, which erupt to replace lost teeth.
See also
References
- ^ a b c d e f g h i j k l m n o p q r s Ten Cate's Oral Histology, Nanci, Elsevier, 2013, pages 70-94
- ^ a b Luan, X. (2005). "Evolution and development of Hertwig's epithelial root sheath". Developmental Dynamics. 235 (5): 1167–1180. doi:10.1002/dvdy.20674. PMC 2734338. PMID 16450392.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d University of Texas Medical Branch.
- ^ Thesleff I, Vaahtokari A, Partanen AM (February 1995). "Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs". The International Journal of Developmental Biology. 39 (1): 35–50. PMID 7626420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thesleff I, Vaahtokari A, Kettunen P, Aberg T (1995). "Epithelial-mesenchymal signaling during tooth development". Connective Tissue Research. 32 (1–4): 9–15. doi:10.3109/03008209509013700. PMID 7554939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ross, Michael H.; Kaye, Gordon I.; Pawlina, Wojciech (2003). Histology: a text and atlas: with cell and molecular biology (4th ed.). Hagerstwon, MD: Lippincott Williams & Wilkins. p. 453. ISBN 978-0-683-30242-4.
- ^ Ash, Major M.; Nelson, Stanley J. (2003). Wheeler's dental anatomy, physiology, and occlusion. Philadelphia: W.B. Saunders. pp. 32, 45, and 53. ISBN 978-0-7216-9382-8.
- ^ University of Southern California School of Dentistry, The Bell Stage: Image 26 found here [1][dead link].
- ^ Barbara Young, Paul R. Wheater (2006). Wheaters Functional Histology. Elsevier Health Sciences. p. 255. ISBN 978-0-443-06850-8.
- ^ University of Southern California School of Dentistry, The Bell Stage: Image 30 found here [2][dead link].
- ^ Ross, Kaye, and Pawlina, Histology: a text and atlas, p. 444.
- ^ Illustrated Dental Embryology, Histology, and Anatomy, Bath-Balogh and Fehrenbach, Elsevier, 2011, page 58-59
- ^ Ten Cate's Oral Histology, Nanci, Elsevier, 2013, page 135
- ^ Ross, Kaye, and Pawlina, Histology: Text and Atlas, p. 445.
- ^ Ross, Kaye, and Pawlina, Histology: Text and Atlas, p. 447.
- ^ a b Illustrated Dental Embryology, Histology, and Anatomy, Bath-Balogh and Fehrenbach, Elsevier, 2011, page 75
- ^ a b c d Cate, Oral Histology, p. 128-139.
- ^ Summitt, Fundamentals of Operative Dentistry, p. 13.
- ^ Summitt, Fundamentals of Operative Dentistry, p. 183.
- ^ Johnson, Biology of the Human Dentition, p. 183.
- ^ a b c d e f g h i j Cate, Oral Histology, p. 236-248.
- ^ Luan X, Ito Y, Diekwisch TG (May 2006). "Evolution and Development of Hertwig's Epithelial Root Sheath". Developmental Dynamics. 235 (5): 1167–80. doi:10.1002/dvdy.20674. PMC 2734338. PMID 16450392.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ross, Kaye, and Pawlina, Histology: Text and Atlas, p. 453.
- ^ a b Ross, Kaye, and Pawlina, Histology: Text and Atlas, p. 452.
- ^ Riolo and Avery, Essentials for Orthodontic Practice, p. 142.
- ^ Harris, Craniofacial Growth and Development, pp. 1–3.
- ^ Harris, Craniofacial Growth and Development, p. 3.
- ^ Harris, Craniofacial Growth and Development, p. 5.
- ^ The American Dental Association, Tooth Eruption Charts found here [3].
- ^ Ash, Major M.; Nelson, Stanley J. (2003). Wheeler's dental anatomy, physiology, and occlusion. Philadelphia: W.B. Saunders. pp. 38 and 41. ISBN 978-0-7216-9382-8.
- ^ Ash, Major M.; Nelson, Stanley J. (2003). Wheeler's dental anatomy, physiology, and occlusion. Philadelphia: W.B. Saunders. p. 38. ISBN 978-0-7216-9382-8.
- ^ WebMd, Dental Health: Your Child's Teeth found here [4].
- ^ Ash, Major M.; Nelson, Stanley J. (2003). Wheeler's dental anatomy, physiology, and occlusion. Philadelphia: W.B. Saunders. p. 41. ISBN 978-0-7216-9382-8.
- ^ Monthly Microscopy Explorations, Exploration of the Month: January 1998 .
- ^ Health Hawaii, Primary Teeth: Importance and Care found here [5].
- ^ American Academy of Periodontology, Oral Health Information for the Public found here [6].
- ^ Ash, Major M.; Nelson, Stanley J. (2003). Wheeler's dental anatomy, physiology, and occlusion. Philadelphia: W.B. Saunders. p. 53. ISBN 978-0-7216-9382-8.
- ^ Armstrong WG (September 1968). "Origin and nature of the acquired pellicle". Proceedings of the Royal Society of Medicine. 61 (9): 923–30. PMC 1902619. PMID 5679017.
- ^ Darling AI (July 1943). "The Distribution of the Enamel Cuticle and Its Significance". Proceedings of the Royal Society of Medicine. 36 (9): 499–502. PMC 1998608. PMID 19992694.
- ^ The American Dental Hygiene Association, Nutritional Factors in Tooth Development found here [7].
- ^ The American Dental Hygiene Association, Table II. Effects of nutrient deficiencies on tooth development found here [8].
- ^ http://celiac.nih.gov/PDF/Dental_Enamel_Defects_508.pdf
- ^ Millett, Declan T.; Richard Welbury (2000). Orthodontics and Paediatric Dentistry. Elsevier Health Sciences. ISBN 0-443-06287-0.
- ^ Neville, Damm, Allen, and Bouquot, Oral & Maxillofacial Pathology, p. 70.
- ^ Kahn, Basic Oral & Maxillofacial Pathology, p. 49.
- ^ Neville, Damm, Allen, and Bouquot, Oral & Maxillofacial Pathology, p. 86.
- ^ Neville, Damm, Allen, and Bouquot, Oral & Maxillofacial Pathology, p. 99.
- ^ Fraser GJ, Hulsey CD, Bloomquist RF, Uyesugi K, Manley NR, Streelman JT (February 2009). Jernvall, Jukka (ed.). "An Ancient Gene Network Is Co-opted for Teeth on Old and New Jaws". PloS Biology. 7 (2): e31. doi:10.1371/journal.pbio.1000031. PMC 2637924. PMID 19215146.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Fraser GJ, Bloomquist RF, Streelman JT (2008). "A periodic pattern generator for dental diversity". BMC Biology. 6: 32. doi:10.1186/1741-7007-6-32. PMC 2496899. PMID 18625062.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Dassule HR, Lewis P, Bei M, Maas R, McMahon AP (November 2000). "Sonic hedgehog regulates growth and morphogenesis of the tooth". Development. 127 (22): 4775–85. PMID 11044393.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I (December 2006). "Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling". Proceedings of the National Academy of Sciences of the United States of America. 103 (49): 18627–32. doi:10.1073/pnas.0607289103. PMC 1693713. PMID 17121988.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Christensen LR, Møllgård K, Kjaer I, Janas MS (September 1993). "Immunocytochemical demonstration of nerve growth factor receptor (NGF-R) in developing human fetal teeth". Anatomy and Embryology. 188 (3): 247–55. doi:10.1007/BF00188216. PMID 8250280.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mitsiadis TA, Dicou E, Joffre A, Magloire H (January 1992). "Immunohistochemical localization of nerve growth factor (NGF) and NGF receptor (NGF-R) in the developing first molar tooth of the rat". Differentiation. 49 (1): 47–61. doi:10.1111/j.1432-0436.1992.tb00768.x. PMID 1320577.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mitsiadis TA, Dicou E, Joffre A, Magloire H. (2001). "歯胚形成を助けるNGFシグナルはp75を介して伝達される". Journal of the Kyushu Dental Society (in Japanese). 55 (6): 347–355. doi:10.2504/kds.55.347.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Amano O, Bringas P, Takahashi I; et al. (November 1999). "Nerve growth factor (NGF) supports tooth morphogenesis in mouse first branchial arch explants". Developmental Dynamics. 216 (3): 299–310. doi:10.1002/(SICI)1097-0177(199911)216:3<299::AID-DVDY8>3.0.CO;2-B. PMID 10590481.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Cobourne MT, Hardcastle Z, Sharpe PT (November 2001). "Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ". Journal of Dental Research. 80 (11): 1974–9. doi:10.1177/00220345010800110501. PMID 11759005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Nakatomi M, Morita I, Eto K, Ota MS (May 2006). "Sonic hedgehog signaling is important in tooth root development". Journal of Dental Research. 85 (5): 427–31. doi:10.1177/154405910608500506. PMID 16632755.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Expression of Sonic hedgehog in mouse tooth". Gene expression in tooth by Pekka Nieminen. Retrieved 2009-10-17.
- ^ Toto PD, O'Malley JJ, Grandel ER (1967). "Similarities of keratinization and amelogenesis". Journal of Dental Research. 46 (3): 602–7. doi:10.1177/00220345670460032401. PMID 4165207.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gustafson G, Sundström B (June 1975). "Enamel: morphological considerations". Journal of Dental Research. 54 Spec No B (2 suppl): B114–20. doi:10.1177/00220345750540020301. PMID 1094042.
- ^ Domingues MG, Jaeger MM, Araújo VC, Araújo NS (February 2000). "Expression of cytokeratins in human enamel organ". European Journal of Oral Sciences. 108 (1): 43–7. doi:10.1034/j.1600-0722.2000.00717.x. PMID 10706476.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rosebury, Theodor (1934). "Presence of Iron in Enamel Keratin". Journal of Dental Research. 14 (4): 269–72. doi:10.1177/00220345340140040301.
- ^ Vaahtokari A, Aberg T, Jernvall J, Keränen S, Thesleff I (January 1996). "The enamel knot as a signaling center in the developing mouse tooth". Mechanisms of Development. 54 (1): 39–43. doi:10.1016/0925-4773(95)00459-9. PMID 8808404.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tucker AS; Headon DJ; Schneider P; et al. (November 2000). "Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis". Development. 127 (21): 4691–700. PMID 11023871.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Thesleff I, Keränen S, Jernvall J (August 2001). "Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation". Advances in Dental Research. 15: 14–8. doi:10.1177/08959374010150010401. PMID 12640732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lesot H, Lisi S, Peterkova R, Peterka M, Mitolo V, Ruch JV (August 2001). "Epigenetic signals during odontoblast differentiation". Advances in Dental Research. 15: 8–13. doi:10.1177/08959374010150012001. PMID 12640731.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Townsend G, Richards L, Hughes T (May 2003). "Molar intercuspal dimensions: genetic input to phenotypic variation". Journal of Dental Research. 82 (5): 350–5. doi:10.1177/154405910308200505. PMID 12709500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Keith A (1913). "Problems relating to the Teeth of the Earlier Forms of Prehistoric Man". Proceedings of the Royal Society of Medicine. 6 (Odontol Sect): 103–124. PMC 2005996. PMID 19977113.
- ^ "Ovarian teratoma (dermoid) with teeth". Doctor T's BrokenDown Palace. Retrieved 2009-11-07.
- ^ Lee R (1860). "On the Nature of Ovarian Cysts which contain Teeth, Hair, and Fatty Matter". Medico-Chirurgical Transactions. 43 (2): 93–114. PMC 2147752. PMID 20896161.
- ^ Eccles WM, Hopewell-Smith A (1912). ""Dermoid Teeth," or Teeth developed in Teratomata". Proceedings of the Royal Society of Medicine. 5 (Odontol Sect): 123–139. PMC 2005364. PMID 19976169.
- ^ Smith CJ (November 1967). "A teratoma of the lung containing teeth". Annals of the Royal College of Surgeons of England. 41 (5): 413–22. PMC 2312017. PMID 6061946.
- ^ Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC (September 1998). "Parathyroid hormone-related protein is required for tooth eruption". Proceedings of the National Academy of Sciences of the United States of America. 95 (20): 11846–51. doi:10.1073/pnas.95.20.11846. PMC 21728. PMID 9751753.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kardong, Kenneth V. (1995). Vertebrates: comparative anatomy, function, evolution. McGraw-Hill. pp. 55, 57. ISBN 0-697-21991-7.
- ^ "Ancylostoma duodenale". Nematode.net Genome Sequencing Center. Archived from the original on 2008-05-16. Retrieved 2009-10-27.
- ^ James WW, Wellings AW (November 1943). "The Dental Epithelium and its Significance in Tooth Development". Proceedings of the Royal Society of Medicine. 37 (1): 1–6.12. PMC 2180846. PMID 19992735.
- ^ Koussoulakou DS, Margaritis LH, Koussoulakos SL (2009). "A Curriculum Vitae of Teeth: Evolution, Generation, Regeneration". International Journal of Biological Sciences. 5 (3): 226–43. doi:10.7150/ijbs.5.226. PMC 2651620. PMID 19266065.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salazar-Ciudad I, Jernvall J (June 2002). "A gene network model accounting for development and evolution of mammalian teeth". Proceedings of the National Academy of Sciences of the United States of America. 99 (12): 8116–20. doi:10.1073/pnas.132069499. PMC 123030. PMID 12048258.
- ^ Gregory W.K. (1920). "The Origin and Evolution of the Human Dentition : A Palaeontological Review. Part I". J Dent Res. 2 (1): 89–183. doi:10.1177/00220345200020011101.
- ^ Gregory W.K. (1921). "The Origin and Evolution of the Human Dentition : A Palaeontological Review. Part II". J Dent Res. 3 (1): 87–228. doi:10.1177/00220345210030011101.
- ^ Sander Kranenbarg. "Skeletal tissue differentiation in fish". Wageninger University. Retrieved 2009-10-24.
- ^ Owen, Richard (1859). The principal forms of the skeleton and the teeth as the basis for a system of natural history and comparativa anatomy. Houlston and Wright. Retrieved 24 October 2009.[page needed]
- ^ Dave Abbott, Sharks, found here [9].
- ^ Boyne PJ (1970). "Study of the chronologic development and eruption of teeth in elasmobranchs". Journal of Dental Research. 49 (3): 556–60. doi:10.1177/00220345700490031501. PMID 5269110.
- ^ Sasagawa I (June 1989). "The fine structure of initial mineralisation during tooth development in the gummy shark, Mustelus manazo, Elasmobranchia". Journal of Anatomy. 164: 175–87. PMC 1256608. PMID 2606790.
- ^ Jason Buchheim, A Quick Course in Ichthyology, found here [10].
- ^ Michael E. Williams, Jaws: The early years, found here [11].
- ^ Sire JY, Delgado SC, Girondot M (2008). "Hen's teeth with enamel cap: from dream to impossibility". BMC Evolutionary Biology. 8: 246. doi:10.1186/1471-2148-8-246. PMC 2542379. PMID 18775069.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Class Mammalia, Order Tubulidentata, Family Orycteropodidae, Species Orycteropus afer," University of Michigan Museum of Zoology. Page accessed November 16, 2009.
- ^ Frandson and Spurgeon, Anatomy and Physiology of Farm Animals., p. 305.
- ^ Caceci. Veterinary Histology with subtitle "Digestive System: Oral Cavity" found here [12].
- ^ Fejerskov O (March 1979). "Human dentition and experimental animals". Journal of Dental Research. 58 (Spec Issue B): 725–34. doi:10.1177/002203457905800224011. PMID 105027.
- ^ Randall-Bowman's April 2004 "Gummed Out: Young Horses Lose Many Teeth, Vet Says." See reference here [13][dead link].
- ^ Encarta
Additional references
- Abbott, Dave. "Sharks". 2000. Page accessed January 7, 2006.
- The American Academy of Periodontology. "Oral Health Information for the Public". Retrieved April 10, 2014.
- The American Dental Association. "Tooth Eruption Charts". Retrieved April 10, 2014.
- The American Dental Hygiene Association. "Nutritional Factors in Tooth Development". Retrieved December 10, 2005.
- The American Dental Hygiene Association. "Table II. Effects of nutrient deficiencies on tooth development". Retrieved December 10, 2005.
- Ash, Major M. and Stanley J. Nelson. Wheeler’s Dental Anatomy, Physiology, and Occlusion. 8th edition. 2003. ISBN 0-7216-9382-2.
- Buchheim, Jason. "A Quick Course in Ichthyology". Page accessed January 7, 2006.
- Cate, A.R. Ten. Oral Histology: development, structure, and function. 5th ed. 1998. ISBN 0-8151-2952-1.
- Caceci, Thomas. Veterinary Histology with subtitle "Digestive System: Oral Cavity". Retrieved December 15, 2005.
- Frandson, R.D. & T.L. Spurgeon, 1992. Anatomy and Physiology of Farm Animals. 5th edition. Philadelphia, Lea & Febiger. ISBN 0-8121-1435-3.
- Harris, Edward F. Craniofacial Growth and Development. In the section entitled "Tooth Eruption." 2002.
- Health Hawaii. "Primary Teeth: Importance and Care". Retrieved December 12, 2005.
- Johnson, Clarke. "Biology of the Human Dentition". 1998.
- Kahn, Michael A. Basic Oral and Maxillofacial Pathology. Volume 1. 2001.
- Monthly Microscopy Explorations "Exploration of the Month: January 1998".
- Neville, B.W., Douglas Damm, Carl Allen, Jerry Bouquot. Oral & Maxillofacial Pathology. 2nd edition. 2002. ISBN 0-7216-9003-3.
- Riolo, Michael L. and James K. Avery. Essentials for Orthodontic Practice. 1st edition. 2003. ISBN 0-9720546-0-X.
- Ross, Michael H., Gordon I. Kaye, and Wojciech Pawlina. Histology: a text and atlas. 4th edition. 2003. ISBN 0-683-30242-6.
- Summitt, James B., J. William Robbins, and Richard S. Schwartz. Fundamentals of Operative Dentistry: A Contemporary Approach. 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc. 2001. ISBN 0-86715-382-2.
- University of Southern California School of Dentistry[dead link]. "The Bell Stage: Image 26"[dead link]. Retrieved December 11, 2005.
- University of Southern California School of Dentistry[dead link]. "The Bell Stage: Image 30"[dead link]. Retrieved December 11, 2005.
- University of Texas Medical Branch. "Lab Exercises: Tooth development"
- Williams, Michael E. "Jaws: The early years". 1992. Page accessed January 7, 2006.
- WebMd. "Dental Health: Your Child's Teeth". Retrieved December 12, 2005.
- <Please add first missing authors to populate metadata.> (1995). "Odontogenesis". The International Journal of Developmental Biology. 39, N° 1. Ijdb.ehu.es. Retrieved 2011-01-20.
External links
- Bio-anthropology and ancient DNA
- Database on the expression of different genes in the developing tooth.
- Embryology at UNSW Notes/skin4a - Integumentary Development Tooth, by Dr. Mark Hill
- Veterinary Histology by Dr. Thomas Caceci.
- Teeth Eruption Timetable on Cleveland Clinic
