Adult stem cell: Difference between revisions
m →Clinical applications: Task 7: replace et al. in author/editor parameters with |display-authors=etal or |display-editors=etal; |
→Adult stem cell therapies: added MSC modifier with ref |
||
| Line 105: | Line 105: | ||
==Adult stem cell therapies== |
==Adult stem cell therapies== |
||
{{main|Stem cell treatments}} |
{{main|Stem cell treatments}} |
||
The therapeutic potential of adult stem cells is the focus of much scientific research, due to their ability to be harvested from the patient.<ref name="pmid17550719">{{cite journal|last=Liao|first=YH|author2=Verchere, CB|author3= Warnock, GL|title=Adult stem or progenitor cells in treatment for type 1 diabetes: current progress.|journal=Canadian journal of surgery. Journal canadien de chirurgie|date=April 2007|volume=50|issue=2|pages=137–42|pmid=17550719|pmc=2384257}}</ref><ref name="pmid17671448">{{cite journal|last=Mimeault|first=M|author2=Hauke, R|author3= Batra, S K|title=Stem Cells: A Revolution in Therapeutics—Recent Advances in Stem Cell Biology and Their Therapeutic Applications in Regenerative Medicine and Cancer Therapies|journal=Clinical Pharmacology & Therapeutics|date=1 August 2007|volume=82|issue=3|pages=252–264|doi=10.1038/sj.clpt.6100301|pmid=17671448}}</ref><ref name="pmid17498520">{{cite journal|last=Christoforou|first=N|author2=Gearhart, JD|title=Stem cells and their potential in cell-based cardiac therapies.|journal=Progress in cardiovascular diseases|date=May–Jun 2007|volume=49|issue=6|pages=396–413|pmid=17498520|doi=10.1016/j.pcad.2007.02.006}}</ref> In common with embryonic stem cells, adult stem cells have the ability to [[Cellular differentiation|differentiate]] into more than one cell type, but unlike the former they are often restricted to certain types or "lineages". The ability of a differentiated stem cell of one lineage to produce cells of a different lineage is called [[transdifferentiation]]. Some types of adult stem cells are more capable of transdifferentiation than others, but for many there is no evidence that such a transformation is possible. Consequently, adult stem therapies require a stem cell source of the specific lineage needed, and harvesting and/or culturing them up to the numbers required is a challenge.<ref>{{ cite journal | doi = 10.1146/annurev.cellbio.19.111301.143037 | last1 = Raff | first1 = M | title = Adult stem cell plasticity: Fact or Artifact? |journal= Annual Review of Cell and Developmental Biology |volume= 19 | pages = 1–22 | year = 2003 | pmid = 14570561 }}</ref><ref>{{ cite journal | url=http://www.sciencemag.org/cgi/content/full/316/5830/1422b | last1=Smith | first1=S | last2=Neaves | first2=W | last3=Teitelbaum | first3=S | last4=Prentice | first4=D. A. | last5=Tarne | first5=G. |title=Adult Versus Embryonic Stem Cells: Treatments | journal= Science | date= 8 June 2007 |volume= 316 |issue= 5830 |pages=1422–1423 | pmid=17556566 | doi=10.1126/science.316.5830.1422b }}</ref> |
The therapeutic potential of adult stem cells is the focus of much scientific research, due to their ability to be harvested from the patient.<ref name="pmid17550719">{{cite journal|last=Liao|first=YH|author2=Verchere, CB|author3= Warnock, GL|title=Adult stem or progenitor cells in treatment for type 1 diabetes: current progress.|journal=Canadian journal of surgery. Journal canadien de chirurgie|date=April 2007|volume=50|issue=2|pages=137–42|pmid=17550719|pmc=2384257}}</ref><ref name="pmid17671448">{{cite journal|last=Mimeault|first=M|author2=Hauke, R|author3= Batra, S K|title=Stem Cells: A Revolution in Therapeutics—Recent Advances in Stem Cell Biology and Their Therapeutic Applications in Regenerative Medicine and Cancer Therapies|journal=Clinical Pharmacology & Therapeutics|date=1 August 2007|volume=82|issue=3|pages=252–264|doi=10.1038/sj.clpt.6100301|pmid=17671448}}</ref><ref name="pmid17498520">{{cite journal|last=Christoforou|first=N|author2=Gearhart, JD|title=Stem cells and their potential in cell-based cardiac therapies.|journal=Progress in cardiovascular diseases|date=May–Jun 2007|volume=49|issue=6|pages=396–413|pmid=17498520|doi=10.1016/j.pcad.2007.02.006}}</ref> In common with embryonic stem cells, adult stem cells have the ability to [[Cellular differentiation|differentiate]] into more than one cell type, but unlike the former they are often restricted to certain types or "lineages". The ability of a differentiated stem cell of one lineage to produce cells of a different lineage is called [[transdifferentiation]]. Some types of adult stem cells are more capable of transdifferentiation than others, but for many there is no evidence that such a transformation is possible. Consequently, adult stem therapies require a stem cell source of the specific lineage needed, and harvesting and/or culturing them up to the numbers required is a challenge.<ref>{{ cite journal | doi = 10.1146/annurev.cellbio.19.111301.143037 | last1 = Raff | first1 = M | title = Adult stem cell plasticity: Fact or Artifact? |journal= Annual Review of Cell and Developmental Biology |volume= 19 | pages = 1–22 | year = 2003 | pmid = 14570561 }}</ref><ref>{{ cite journal | url=http://www.sciencemag.org/cgi/content/full/316/5830/1422b | last1=Smith | first1=S | last2=Neaves | first2=W | last3=Teitelbaum | first3=S | last4=Prentice | first4=D. A. | last5=Tarne | first5=G. |title=Adult Versus Embryonic Stem Cells: Treatments | journal= Science | date= 8 June 2007 |volume= 316 |issue= 5830 |pages=1422–1423 | pmid=17556566 | doi=10.1126/science.316.5830.1422b }}</ref> Additionally, cues from the immediate environment (including how stiff or porous the surrounding structure/[[extracellular matrix]] is) can alter or enhance the fate and differentiation of the stem cells.<ref>{{cite journal|last1=Huang, C et al.|title=Environmental physical cues determine the lineage specification of mesenchymal stem cells|journal=Biochim Biophys Acta|date=2015|volume=1850|pages=1261-6|doi=http://dx.doi.org/10.1016/j.bbagen.2015.02.011|pmid=25727396}}</ref> |
||
===Sources=== |
===Sources=== |
||
Revision as of 04:03, 4 May 2015
| Adult stem cell | |
|---|---|
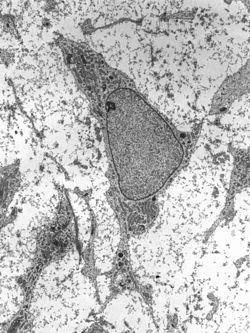 Transmission electron micrograph of an adult stem cell displaying typical ultrastructural characteristics. | |
| Details | |
| Identifiers | |
| Latin | Cellula praecursoria |
| MeSH | D053687 |
| TH | H1.00.01.0.00035 |
| Anatomical terminology | |
Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells (from Greek Σωματικóς, meaning of the body), they can be found in juvenile as well as adult animals and human bodies.
Scientific interest in adult stem cells is centered on their ability to divide or self-renew indefinitely, and generate all the cell types of the organ from which they originate, potentially regenerating the entire organ from a few cells. Unlike embryonic stem cells, the use of human adult stem cells in research and therapy is not considered to be controversial, as they are derived from adult tissue samples rather than human 5 day old embryos generated by IVF (in vitro fertility) clinics designated for scientific research. They have mainly been studied in humans and model organisms such as mice and rats.

Defining properties
A stem cell possesses two properties:
- Self-renewal, which is the ability to go through numerous cycles of cell division while still maintaining its undifferentiated state.
- multipotency or multidifferentiative potential, which is the ability to generate progeny of several distinct cell types, (for example glial cells and neurons) as opposed to unipotency, which is the term for cells that are restricted to producing a single-cell type. However, some researchers do not consider multipotency to be essential, and believe that unipotent self-renewing stem cells can exist.[1] These properties can be illustrated with relative ease in vitro, using methods such as clonogenic assays, where the progeny of a single cell is characterized. However, it is known that in vitro cell culture conditions can alter the behavior of cells, proving that a particular subpopulation of cells possesses stem cell properties in vivo is challenging, and so considerable debate exists as to whether some proposed stem cell populations in the adult are indeed stem cells.
Lineage
To ensure the safety of others, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives us a rise to two identical daughter cells, both endowed with stem cell properties, whereas asymmetric such division produces only one of those stem cells and a progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before finally differentiating into a mature cell. It is believed that the molecular distinction between symmetric and asymmetric divisions lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells.
Multidrug resistance
Adult stem cells express transporters of the ATP-binding cassette family that actively pump a diversity of organic molecules out of the cell.[2] Many pharmaceuticals are exported by these transporters conferring multidrug resistance onto the cell. This complicates the design of drugs, for instance neural stem cell targeted therapies for the treatment of clinical depression.
Signaling pathways
Adult stem cell research has been focused on uncovering the general molecular mechanisms that control their self-renewal and differentiation.
- The Notch pathway has been known to developmental biologists for decades. Its role in control of stem cell proliferation has now been demonstrated for several cell types including haematopoietic, neural, and mammary[3] stem cells.
- These developmental pathways are also strongly implicated as stem cell regulators.[4]
Plasticity/Adult stem cell pluripotency
Discoveries in recent years have suggested that adult stem cells might have the ability to differentiate into cell types from different germ layers. For instance, neural stem cells from the brain, which are derived from ectoderm, can differentiate into ectoderm, mesoderm, and endoderm.[5] Stem cells from the bone marrow, which is derived from mesoderm, can differentiate into liver, lung, GI tract and skin, which are derived from endoderm and mesoderm.[6] This phenomenon is referred to as stem cell transdifferentiation or plasticity. It can be induced by modifying the growth medium when stem cells are cultured in vitro or transplanting them to an organ of the body different from the one they were originally isolated from. There is yet no consensus among biologists on the prevalence and physiological and therapeutic relevance of stem cell plasticity. More recent findings suggest that pluripotent stem cells may reside in blood and adult tissues in a dormant state.[7] These cells are referred to as "Blastomere Like Stem Cells" (Am Surg. 2007 Nov;73:1106-10) and "very small embryonic like" - "VSEL" stem cells, and display pluripotency in vitro.[7] As BLSC's and VSEL cells are present in virtually all adult tissues, including lung, brain, kidneys, muscles, and pancreas[8] Co-purification of BLSC's and VSEL cells with other populations of adult stem cells may explain the apparent pluripotency of adult stem cell populations. However, recent studies have shown that both human and murine VSEL cells lack stem cell characteristics and are not pluripotent.[9][10][11][12]
Types
Hematopoietic stem cells
Hematopoietic stem cells are found in the bone marrow and give rise to all the blood cell types.
Mammary stem cells
Mammary stem cells provide the source of cells for growth of the mammary gland during puberty and gestation and play an important role in carcinogenesis of the breast.[13] Mammary stem cells have been isolated from human and mouse tissue as well as from cell lines derived from the mammary gland. Single such cells can give rise to both the luminal and myoepithelial cell types of the gland, and have been shown to have the ability to regenerate the entire organ in mice.[13]
Intestinal stem cells
Intestinal stem cells divide continuously throughout life and use a complex genetic program to produce the cells lining the surface of the small and large intestines.[14] Intestinal stem cells reside near the base of the stem cell niche, called the crypts of Lieberkuhn. Intestinal stem cells are probably the source of most cancers of the small intestine and colon.[15]
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are of stromal origin and may differentiate into a variety of tissues. MSCs have been isolated from placenta, adipose tissue, lung, bone marrow and blood, Wharton's jelly from the umbilical cord,[16] and teeth (perivascular niche of dental pulp and periodontal ligament).[17] MSCs are attractive for clinical therapy due to their ability to differentiate, provide trophic support, and modulate innate immune response.[16]
Endothelial stem cells
Endothelial stem cells are one of the three types of multipotent stem cells found in the bone marrow. They are a rare and controversial group with the ability to differentiate into endothelial cells, the cells that line blood vessels.
Neural stem cells
The existence of stem cells in the adult brain has been postulated following the discovery that the process of neurogenesis, the birth of new neurons, continues into adulthood in rats.[18] The presence of stem cells in the mature primate brain was first reported in 1967.[19] It has since been shown that new neurons are generated in adult mice, songbirds and primates, including humans. Normally, adult neurogenesis is restricted to two areas of the brain – the subventricular zone, which lines the lateral ventricles, and the dentate gyrus of the hippocampal formation.[20] Although the generation of new neurons in the hippocampus is well established, the presence of true self-renewing stem cells there has been debated.[21] Under certain circumstances, such as following tissue damage in ischemia, neurogenesis can be induced in other brain regions, including the neocortex.
Neural stem cells are commonly cultured in vitro as so called neurospheres – floating heterogeneous aggregates of cells, containing a large proportion of stem cells.[22] They can be propagated for extended periods of time and differentiated into both neuronal and glia cells, and therefore behave as stem cells. However, some recent studies suggest that this behaviour is induced by the culture conditions in progenitor cells, the progeny of stem cell division that normally undergo a strictly limited number of replication cycles in vivo.[23] Furthermore, neurosphere-derived cells do not behave as stem cells when transplanted back into the brain.[24]
Neural stem cells share many properties with haematopoietic stem cells (HSCs). Remarkably, when injected into the blood, neurosphere-derived cells differentiate into various cell types of the immune system.[25]
Olfactory adult stem cells
Olfactory adult stem cells have been successfully harvested from the human olfactory mucosa cells, which are found in the lining of the nose and are involved in the sense of smell.[26] If they are given the right chemical environment these cells have the same ability as embryonic stem cells to develop into many different cell types. Olfactory stem cells hold the potential for therapeutic applications and, in contrast to neural stem cells, can be harvested with ease without harm to the patient. This means they can be easily obtained from all individuals, including older patients who might be most in need of stem cell therapies.
Neural crest stem cells
Hair follicles contain two types of stem cells, one of which appears to represent a remnant of the stem cells of the embryonic neural crest. Similar cells have been found in the gastrointestinal tract, sciatic nerve, cardiac outflow tract and spinal and sympathetic ganglia. These cells can generate neurons, Schwann cells, myofibroblast, chondrocytes and melanocytes.[27][28]
Testicular cells
Multipotent stem cells with a claimed equivalency to embryonic stem cells have been derived from spermatogonial progenitor cells found in the testicles of laboratory mice by scientists in Germany[29][30][31] and the United States,[32][33][34][35] and, a year later, researchers from Germany and the United Kingdom confirmed the same capability using cells from the testicles of humans.[36] The extracted stem cells are known as human adult germline stem cells (GSCs)[37]
Multipotent stem cells have also been derived from germ cells found in human testicles.[38]
Adult stem cell therapies
The therapeutic potential of adult stem cells is the focus of much scientific research, due to their ability to be harvested from the patient.[39][40][41] In common with embryonic stem cells, adult stem cells have the ability to differentiate into more than one cell type, but unlike the former they are often restricted to certain types or "lineages". The ability of a differentiated stem cell of one lineage to produce cells of a different lineage is called transdifferentiation. Some types of adult stem cells are more capable of transdifferentiation than others, but for many there is no evidence that such a transformation is possible. Consequently, adult stem therapies require a stem cell source of the specific lineage needed, and harvesting and/or culturing them up to the numbers required is a challenge.[42][43] Additionally, cues from the immediate environment (including how stiff or porous the surrounding structure/extracellular matrix is) can alter or enhance the fate and differentiation of the stem cells.[44]
Sources
Pluripotent stem cells, i.e. cells that can give rise to any fetal or adult cell type, can be found in a number of tissues, including umbilical cord blood.[45] Using genetic reprogramming, pluripotent stem cells equivalent to embryonic stem cells have been derived from human adult skin tissue.[46][47][48][49][50] Other adult stem cells are multipotent, meaning they are restricted in the types of cell they can become, and are generally referred to by their tissue origin (such as mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell, etc.).[51][52] A great deal of adult stem cell research has focused on investigating their capacity to divide or self-renew indefinitely, and their potential for differentiation.[53] In mice, pluripotent stem cells can be directly generated from adult fibroblast cultures.[54]
Clinical applications
Adult stem cell treatments have been used for many years to successfully treat leukemia and related bone/blood cancers utilizing bone marrow transplants.[55] The use of adult stem cells in research and therapy is not considered as controversial as the use of embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo.
Early regenerative applications of adult stem cells has focused on intravenous delivery of blood progenitors known as Hematopetic Stem Cells (HSC's). CD34+ hematopoietic Stem Cells have been clinically applied to treat various diseases including spinal cord injury,[56] liver cirrhosis [57] and Peripheral Vascular disease.[58] Research has shown that CD34+ hematopoietic Stem Cells are relatively more numerous in men than in women of reproductive age group among spinal cord Injury victims.[59] Other early commercial applications have focused on Mesenchymal Stem Cells (MSCs). For both cell lines, direct injection or placement of cells into a site in need of repair may be the preferred method of treatment, as vascular delivery suffers from a "pulmonary first pass effect" where intravenous injected cells are sequestered in the lungs.[60] Clinical case reports in orthopedic applications have been published. Wakitani has published a small case series of nine defects in five knees involving surgical transplantation of mesenchymal stem cells with coverage of the treated chondral defects.[61] Centeno et al. have reported high field MRI evidence of increased cartilage and meniscus volume in individual human clinical subjects as well as a large n=227 safety study.[62][63][64][65] Many other stem cell based treatments are operating outside the US, with much controversy being reported regarding these treatments as some feel more regulation is needed as clinics tend to exaggerate claims of success and minimize or omit risks.[66]
First transplanted human organ grown from adult stem cells
In 2008 the first full transplant of a human organ grown from adult stem cells was carried out by Paolo Macchiarini, at the Hospital Clínic of Barcelona on Claudia Castillo, a Colombian female adult whose trachea had collapsed due to tuberculosis. Researchers from the University of Padua, the University of Bristol, and Politecnico di Milano harvested a section of trachea from a donor and stripped off the cells that could cause an immune reaction, leaving a grey trunk of cartilage. This section of trachea was then "seeded" with stem cells taken from Ms. Castillo's bone marrow and a new section of trachea was grown in the laboratory over four days. The new section of trachea was then transplanted into the left main bronchus of the patient.[67][68][69][70][71] Because the stem cells were harvested from the patient's own bone marrow Professor Macchiarini did not think it was necessary for her to be given anti-rejection (immunosuppressive) medication and when the procedure was reported four months later in The Lancet, the patient's immune system was showing no signs of rejecting the transplant.[72]
Adult stem cells and cancer
In recent years, acceptance of the concept of adult stem cells has increased. There is now a hypothesis that stem cells reside in many adult tissues and that these unique reservoirs of cells not only are responsible for the normal reparative and regenerative processes but are also considered to be a prime target for genetic and epigenetic changes, culminating in many abnormal conditions including cancer.[73][74]
Most Recent Research Presented by NIH
- 2012 January 24: Preliminary Report – Stem Cell-Derived Treatments for Eye Disease. This has only been through Phase 1 clinical trials therefore, there is still further research to be done.
- 2012 February 26: Women May Be Able to Make New Eggs: The research conducted showed adult female mice are able to make new eggs. Recent evidence demonstrates that women of reproductive age are also able to make new eggs. This knowledge was used to identify these kinds of cells in adult ovaries that can still divide, be grown in a lab and appear as mature eggs. Further testing must be done to confirm these results which may have the capability of treating infertility.
- 2012 March 27: Arsenic turns stem cells cancerous.
- 2012 September 12: Brains of Deaf Adult Gerbils Respond to Sound after Implant of Human Embryonic Stem Cell-Derived Early Ear Cells: Scientists now hope to refine this technique to treat deafness in humans.
- 2012 October 4: Eggs Generated from Mouse Stem Cells Produce Live Mice: This report shows the necessary steps to generate a mature oocyte from stem cells. The complicated protocol used to conduct these experiments in mice is not practical for use in humans.[75]
See also
News and external links
- NIH Stem Cell Information Resource, resource for stem cell research
- Nature Reports Stem Cells Background information, research advances and debates about stem cell science
- UMDNJ Stem Cell and Regenerative Medicine, provides educational materials and research resources
- Stem cell research pros and cons, Information and resource for stem cell research
- Stem Cell Research at Johns Hopkins University
References
- ^ Mlsna, Lucas J. (2010). "Stem Cell Based Treatments and Novel Considerations for Conscience Clause Legislation". Indiana Health Law Review. 8 (2). United States: Indiana University Robert H. McKinney School of Law: 471–496. ISSN:1549-3199. LCCN:2004212209. OCLC:54703225.
- ^ Chaudhary PM, Roninson IB (July 1991). "Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells". Cell. 66 (1): 85–94. doi:10.1016/0092-8674(91)90141-K. PMID 1712673.
- ^ Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS (2004). "Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells". Breast Cancer Research. 6 (6): R605–15. doi:10.1186/bcr920. PMC 1064073. PMID 15535842.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Beachy PA, Karhadkar SS, Berman DM; Karhadkar; Berman (November 2004). "Tissue repair and stem cell renewal in carcinogenesis". Nature. 432 (7015): 324–31. Bibcode:2004Natur.432..324B. doi:10.1038/nature03100. PMID 15549094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clarke, D. L.; Johansson, CB; Wilbertz, J; Veress, B; Nilsson, E; Karlström, H; Lendahl, U; Frisén, J (2000). "Generalized Potential of Adult Neural Stem Cells". Science. 288 (5471): 1660–3. Bibcode:2000Sci...288.1660C. doi:10.1126/science.288.5471.1660. PMID 10834848.
- ^ Krause, Diane S.; Theise, Neil D.; Collector, Michael I.; Henegariu, Octavian; Hwang, Sonya; Gardner, Rebekah; Neutzel, Sara; Sharkis, Saul J. (2001). "Multi-Organ, Multi-Lineage Engraftment by a Single Bone Marrow-Derived Stem Cell". Cell. 105 (3): 369–77. doi:10.1016/S0092-8674(01)00328-2. PMID 11348593.
- ^ a b Kucia, M; Reca, R; Campbell, F R; Zuba-Surma, E; Majka, M; Ratajczak, J; Ratajczak, M Z (2006). "A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow". Leukemia. 20 (5): 857–69. doi:10.1038/sj.leu.2404171. PMID 16498386.
- ^ Zuba-Surma, Ewa K.; Kucia, Magdalena; Wu, Wan; Klich, Izabela; Lillard, James W.; Ratajczak, Janina; Ratajczak, Mariusz Z. (2008). "Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies". Cytometry Part A. 73A (12): 1116. doi:10.1002/cyto.a.20667.
- ^ Danova-Alt, Ralitza; Heider, Andreas; Egger, Dietmar; Cross, Michael; Alt, Rüdiger; Ivanovic, Zoran (2 April 2012). Ivanovic, Zoran (ed.). "Very Small Embryonic-Like Stem Cells Purified from Umbilical Cord Blood Lack Stem Cell Characteristics". PLoS ONE. 7 (4): e34899. doi:10.1371/journal.pone.0034899. PMC 3318011. PMID 22509366.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Szade, Krzysztof; Bukowska-Strakova, Karolina; Nowak, Witold Norbert; Szade, Agata; Kachamakova-Trojanowska, Neli; Zukowska, Monika; Jozkowicz, Alicja; Dulak, Jozef; Asakura, Atsushi (16 May 2013). Asakura, Atsushi (ed.). "Murine Bone Marrow Lin−Sca-1+CD45− Very Small Embryonic-Like (VSEL) Cells Are Heterogeneous Population Lacking Oct-4A Expression". PLoS ONE. 8 (5): e63329. doi:10.1371/journal.pone.0063329. PMC 3656957. PMID 23696815.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CKF, et al. Stem Cell Reports. Stem Cell Reports. 2013 Jul 23;:1–11. http://www.cell.com/stem-cell-reports/abstract/S2213-6711(13)00050-7?fb_action_ids=10201558251787555&fb_action_types=og.likes
- ^ Miyanishi, Masanori; Mori, Yasuo; Seita, Jun; Chen, James Y.; Karten, Seth; Chan, Charles K.F.; Nakauchi, Hiromitsu; Weissman, Irving L. (31 July 2013). "Do Pluripotent Stem Cells Exist in Adult Mice as Very Small Embryonic Stem Cells?". Stem Cell Reports. 1 (2): 198–208. doi:10.1016/j.stemcr.2013.07.001. PMC 3757755. PMID 24052953.
- ^ a b Liu S, Dontu G, Wicha MS (2005). "Mammary stem cells, self-renewal pathways, and carcinogenesis". Breast Cancer Research. 7 (3): 86–95. doi:10.1186/bcr1021. PMC 1143566. PMID 15987436.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18808327, please use {{cite journal}} with
|pmid=18808327instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19092804, please use {{cite journal}} with
|pmid=19092804instead. - ^ a b Phinney DG, Prockop DJ (November 2007). "Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views". Stem Cells. 25 (11): 2896–902. doi:10.1634/stemcells.2007-0637. PMID 17901396.
- ^ Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (August 2005). "The efficacy of mesenchymal stem cells to regenerate and repair dental structures". Orthod Craniofac Res. 8 (3): 191–9. doi:10.1111/j.1601-6343.2005.00331.x. PMID 16022721.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Altman J, Das GD (June 1965). "Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats". The Journal of Comparative Neurology. 124 (3): 319–35. doi:10.1002/cne.901240303. PMID 5861717.
- ^ Lewis PD (March 1968). "Mitotic activity in the primate subependymal layer and the genesis of gliomas". Nature. 217 (5132): 974–5. Bibcode:1968Natur.217..974L. doi:10.1038/217974a0. PMID 4966809.
- ^ Alvarez-Buylla A, Seri B, Doetsch F (April 2002). "Identification of neural stem cells in the adult vertebrate brain". Brain Research Bulletin. 57 (6): 751–8. doi:10.1016/S0361-9230(01)00770-5. PMID 12031271.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bull ND, Bartlett PF (November 2005). "The adult mouse hippocampal progenitor is neurogenic but not a stem cell". The Journal of Neuroscience. 25 (47): 10815–21. doi:10.1523/JNEUROSCI.3249-05.2005. PMID 16306394.
- ^ Reynolds BA, Weiss S; Weiss (March 1992). "Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system". Science. 255 (5052): 1707–10. Bibcode:1992Sci...255.1707R. doi:10.1126/science.1553558. PMID 1553558.
- ^ Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (December 2002). "EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells". Neuron. 36 (6): 1021–34. doi:10.1016/S0896-6273(02)01133-9. PMID 12495619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marshall GP, Laywell ED, Zheng T, Steindler DA, Scott EW (March 2006). "In vitro-derived "neural stem cells" function as neural progenitors without the capacity for self-renewal". Stem Cells. 24 (3): 731–8. doi:10.1634/stemcells.2005-0245. PMID 16339644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL; Rietze; Reynolds; Cristina Magli; Vescovi (January 1999). "Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo". Science. 283 (5401): 534–7. Bibcode:1999Sci...283..534B. doi:10.1126/science.283.5401.534. PMID 9915700.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Murrell W; Féron F; Wetzig A; et al. (June 2005). "Multipotent stem cells from adult olfactory mucosa". Developmental Dynamics. 233 (2): 496–515. doi:10.1002/dvdy.20360. PMID 15782416.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sieber-Blum M, Hu Y (December 2008). "Epidermal neural crest stem cells (EPI-NCSC) and pluripotency". Stem Cell Rev. 4 (4): 256–60. doi:10.1007/s12015-008-9042-0. PMID 18712509.
- ^ Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ (August 2002). "Neural Crest Stem Cells Persist in the Adult Gut but Undergo Changes in Self-Renewal, Neuronal Subtype Potential, and Factor Responsiveness". Neuron. 35 (4): 657–69. doi:10.1016/S0896-6273(02)00827-9. PMC 2728576. PMID 12194866.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Testicle cells may aid research". BBC. 25 March 2006.
- ^ CBS/Associated Press (24 March 2006). "Study: Mice Testes Act Like Stem Cells". CBS.
- ^ Rick Weiss (25 March 2006). "Embryonic Stem Cell Success". Washington Post.
- ^ "Promising New Source Of Stem Cells: Mouse Testes Produce Wide Range Of Tissue Types". Science Daily. 24 September 2007.
- ^ Barbara Miller (20 September 2007). "Testicles yield stem cells in science breakthrough". Australian Broadcasting Corporation.
- ^ J.R. Minkel (19 September 2007). "Testes May Prove Fertile Source of Stem Cells". Scientific American.
- ^ "Stem Cells in Adult Testes Provide Alternative to Embryonic Stem Cells for Organ Regeneration". Cornell University. 20 September 2007.
- ^ Rob Waters (8 October 2008). "Testicle Stem Cells Become Bone, Muscle in German Experiments". Bloomberg.
- ^ Nora Schultz (9 October 2008). "A Source of Men's Stem Cells – Stem cells from human testes could be used for personalized medicine". Technology Review.
- ^ Maggie Fox (Reuters) (2 April 2006). "U.S. Firm Says It Made Stem Cells From Human Testes". Washington Post.
{{cite web}}:|author=has generic name (help) - ^ Liao, YH; Verchere, CB; Warnock, GL (April 2007). "Adult stem or progenitor cells in treatment for type 1 diabetes: current progress". Canadian journal of surgery. Journal canadien de chirurgie. 50 (2): 137–42. PMC 2384257. PMID 17550719.
- ^ Mimeault, M; Hauke, R; Batra, S K (1 August 2007). "Stem Cells: A Revolution in Therapeutics—Recent Advances in Stem Cell Biology and Their Therapeutic Applications in Regenerative Medicine and Cancer Therapies". Clinical Pharmacology & Therapeutics. 82 (3): 252–264. doi:10.1038/sj.clpt.6100301. PMID 17671448.
- ^ Christoforou, N; Gearhart, JD (May–June 2007). "Stem cells and their potential in cell-based cardiac therapies". Progress in cardiovascular diseases. 49 (6): 396–413. doi:10.1016/j.pcad.2007.02.006. PMID 17498520.
- ^ Raff, M (2003). "Adult stem cell plasticity: Fact or Artifact?". Annual Review of Cell and Developmental Biology. 19: 1–22. doi:10.1146/annurev.cellbio.19.111301.143037. PMID 14570561.
- ^ Smith, S; Neaves, W; Teitelbaum, S; Prentice, D. A.; Tarne, G. (8 June 2007). "Adult Versus Embryonic Stem Cells: Treatments". Science. 316 (5830): 1422–1423. doi:10.1126/science.316.5830.1422b. PMID 17556566.
- ^ Huang, C; et al. (2015). "Environmental physical cues determine the lineage specification of mesenchymal stem cells". Biochim Biophys Acta. 1850: 1261–6. doi:http://dx.doi.org/10.1016/j.bbagen.2015.02.011. PMID 25727396.
{{cite journal}}: Check|doi=value (help); Explicit use of et al. in:|last1=(help); External link in|doi= - ^ Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M (2007). "A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues". Leukemia. 21 (5): 860–7. doi:10.1038/sj.leu.2404630. PMID 17344915.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Me too, too – How to make human embryonic stem cells without destroying human embryos". The Economist. 22 November 2007.
- ^ Gina Kolata (22 November 2007). "Man Who Helped Start Stem Cell War May End It". New York Times.
- ^ Gina Kolata (21 November 2007). "Scientists Bypass Need for Embryo to Get Stem Cells". New York Times.
- ^ Anne McIlroy (21 November 2007). "Stem-cell method hailed as 'massive breakthrough'". Globe and Mail. Canada.
- ^ Alice Park (20 November 2007). "A Breakthrough on Stem Cells". Time Magazine.
- ^ Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC (2006). "Review: ex vivo engineering of living tissues with adult stem cells". Tissue Eng. 12 (11): 3007–19. doi:10.1089/ten.2006.12.3007. PMID 17518617.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gimble JM, Katz AJ, Bunnell BA (2007). "Adipose-derived stem cells for regenerative medicine". Circ. Res. 100 (9): 1249–60. doi:10.1161/01.RES.0000265074.83288.09. PMID 17495232.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gardner RL (March 2002). "Stem cells: potency, plasticity and public perception". Journal of Anatomy. 200 (Pt 3): 277–82. doi:10.1046/j.1469-7580.2002.00029.x. PMC 1570679. PMID 12033732.
- ^ Takahashi K, Yamanaka S (2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell. 126 (4): 663–76. doi:10.1016/j.cell.2006.07.024. PMID 16904174.
- ^ Bone Marrow Transplant Retrieved on 21 November 2008
- ^ Srivastava A, Bapat M, Ranade S, Srinivasan V, Murugan P, Manjunath S, Thamaraikannan P, Abraham S (2010). "Autologous Multiple Injections of in Vitro Expanded Autologous Bone Marrow Stem Cells For Cervical Level Spinal Cord Injury - A Case Report". Journal of Stem Cells and Regenerative Medicine.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y,Uchida K, Yamasaki T, Fujii Y, Okita K, Sakaida I (2006). "Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy". Stem Cells. 24 (10): 2292–8. doi:10.1634/stemcells.2005-0542. PMID 16778155.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S,Manjunath SR, Senthilkumar R, Murugan P, Srinivasan VR, Abraham S (2011). "Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience". Cytotherapy. 13 (8): 993–9. doi:10.3109/14653249.2011.579961. PMID 21671823.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dedeepiya V, Rao Y Y, Jayakrishnan G, Parthiban JKBC, Baskar S, Manjunath S, Senthilkumar R and Abraham S (2012). "Index of CD34+ cells and mononuclear cells in the bone marrow of spinal cord injury patients of different age groups- A comparative analysis". Bone Marrow Research. 2012: 1. doi:10.1155/2012/787414. PMC 3398573. PMID 22830032.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Fischer UM; Harting MT; Jimenez F; et al. (June 2009). "Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect". Stem Cells and Development. 18 (5): 683–92. doi:10.1089/scd.2008.0253. PMC 3190292. PMID 19099374.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H (2007). "Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees". Journal of Tissue Engineering and Regenerative Medicine. 1 (1): 74–9. doi:10.1002/term.8. PMID 18038395.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ >>Centeno; et al. "Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells, platelate lysate, and dexamethasome".
- ^ Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (December 2008). "Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells". Medical Hypotheses. 71 (6): 900–8. doi:10.1016/j.mehy.2008.06.042. PMID 18786777.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (2008). "Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells". Pain Physician. 11 (3): 343–53. PMID 18523506.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W (2010). "Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique". Curr Stem Cell Res Ther. 5 (1): 81–93. doi:10.2174/157488810790442796. PMID 19951252.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ >PR Newswire. "The International Society for Stem Cell Research Releases New Guidelines to Shape Future of Stem Cell Therapy Regulation needed as new study reveals clinics exaggerate claims and omit risks".
- ^ "Claudia Castillo: The pioneer's story". The Independent(United Kingdom). 19 November 2008.
- ^ Michael Kahn (18 November 2008). "Woman gets first trachea transplant without drugs". Reuters.
- ^ Kate Devlin (18 November 2008). "British doctors help perform world's first transplant of a whole organ grown in lab". The Daily Telegraph. United Kingdom.
- ^ Tanya Thompson (19 November 2008). "World first as woman gets organ made from stem cells". The Scotsman. UK.
- ^ Jeremy Laurance (19 November 2008). "The medical miracle". The Independent(United Kingdom).
- ^ Rose, David (19 November 2008). "Claudia Castillo gets windpipe tailor-made from her own stem cells". The Times. London: The Times Newspapers Ltd. Retrieved 20 November 2008.
- ^ Bioinfobank FAQ:Stem cells in adult tissues Retrieved on 21 November 2008 [dead link]
- ^ Cogle CR, Guthrie SM, Sanders RC, Allen WL, Scott EW, Petersen BE (August 2003). "An overview of stem cell research and regulatory issues". Mayo Clinic Proceedings. Mayo Clinic. 78 (8): 993–1003. doi:10.4065/78.8.993. PMID 12911047.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Highlights of Stem Cell Research". National Institutes of Health. U. S. Department of Health & Human Services. Retrieved 28 May 2013.
