Plutonium: Difference between revisions
No edit summary |
rvv |
||
| Line 212: | Line 212: | ||
[[uk:Плутоній]] |
[[uk:Плутоній]] |
||
[[zh:钚]] |
[[zh:钚]] |
||
== Compounds == |
|||
sss |
|||
Revision as of 00:11, 1 March 2006
Template:Elementbox header Template:Elementbox series Template:Elementbox periodblock Template:Elementbox appearance Template:Elementbox atomicmass gpm Template:Elementbox econfig Template:Elementbox epershell Template:Elementbox section physicalprop Template:Elementbox phase Template:Elementbox density gpcm3nrt Template:Elementbox densityliq gpcm3mp Template:Elementbox meltingpoint Template:Elementbox boilingpoint Template:Elementbox heatfusion kjpmol Template:Elementbox heatvaporiz kjpmol Template:Elementbox heatcapacity jpmolkat25 Template:Elementbox vaporpressure katpa Template:Elementbox section atomicprop Template:Elementbox crystalstruct Template:Elementbox oxistates Template:Elementbox electroneg pauling Template:Elementbox ionizationenergies1 Template:Elementbox atomicradius pm Template:Elementbox section miscellaneous Template:Elementbox magnetic Template:Elementbox eresist ohmmat0 Template:Elementbox thermalcond wpmkat300k Template:Elementbox thermalexpansion umpmkat25 Template:Elementbox speedofsound rodmpsat20 Template:Elementbox youngsmodulus gpa Template:Elementbox shearmodulus gpa Template:Elementbox poissonratio Template:Elementbox cas number Template:Elementbox isotopes begin |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 238Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 88 y | SF | style="text-align:right;" | - |- | α | style="text-align:right;" | 234U |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 239Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | trace | rowspan="2" style="text-align:right; vertical-align:middle;" | 24.1×103 y | SF | style="text-align:right;" | - |- | α | style="text-align:right;" | 235U |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 240Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 6.5×103 y | SF | style="text-align:right;" | - |- | β | style="text-align:right;" | 240Am |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 241Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 14 y | α | style="text-align:right;" | U |- | SF | style="text-align:right;" | - |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 242Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 3.73×105 y | SF | style="text-align:right;" | - |- | α | style="text-align:right;" | 238U |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | 244Pu | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 8.08×107 y | α | style="text-align:right;" | 240U |- | SF | style="text-align:right;" | - Template:Elementbox isotopes end Template:Elementbox footer

Plutonium is a radioactive, metallic, chemical element. It has the symbol Pu and the atomic number 94. It is the element used in most modern nuclear weapons. The most important, albeit not most stable, isotope of plutonium is 239Pu, with a half-life of 24,110 years.
Notable characteristics
Plutonium is silvery in pure form, but has a yellow tarnish when oxidized. Peculiarly, the metal goes through phases of contraction as its temperature is increased.
The heat given off by alpha particle emission makes plutonium warm to the touch in reasonable quantities; larger amounts can boil water. It displays four ionic oxidation states in aqueous solution:
- PuIII, as Pu3+ (blue lavender)
- PuIV, as Pu4+ (yellow brown)
- PuVI, as PuO22+ (pink orange)
- PuV, as PuO2+ (thought to be pink; this ion is unstable in solution and will disproportionate into Pu4+ and PuO22+; the Pu4+ will then oxidize the remaining PuO2+ to PuO22+, being reduced in turn to Pu3+. Thus, aqueous solutions of plutonium tend over time towards a mixture of Pu3+ and PuO22+.) [1]
Applications
The isotope 239Pu is a key fissile component in modern nuclear weapons, due to its ease of fissioning and availability. The critical mass for an unreflected sphere of plutonium is 16 kg, but through the use of a neutron reflecting tamper the pit of plutonium in a fission bomb is reduced to 10 kg, which is a sphere with a diameter of 10 cm. Complete detonation of plutonium will produce an explosion of 20 kilotons of TNT per kilogram. (See also Nuclear Weapon Design.)
Plutonium could also be used to manufacture radiological weapons or as a (not particularly deadly) poison.
The plutonium isotope 238Pu is an alpha emitter with a half-life of 87 years. These characteristics make it well suited for electrical power generation for devices which must function without direct maintenance for timescales approximating a human lifetime. It is therefore used in radioisotope thermoelectric generators such as those powering the Galileo and Cassini space probes; earlier versions of the same technology powered seismic experiments on the Apollo Moon missions.
238Pu has been used successfully to power artificial heart pacemakers, to reduce the risk of repeated surgery. It has been largely replaced by lithium-based batteries recharged by induction, but as of 2003 there were somewhere between 50 and 100 plutonium-powered pacemakers still implanted and functioning in living patients.
History
Initially predicted by Walter Russell, the production of plutonium and neptunium by bombarding uranium-238 with neutrons was predicted in 1940 by two teams working independently: Edwin M. McMillan and Philip Abelson at Berkeley Radiation Laboratory at the University of California, Berkeley and by Norman Feather & Egon Bretscher at the Cavendish Laboratory at University of Cambridge. Coincidentally both teams proposed the same names to follow on from uranium, like the sequence of the outer planets.
Plutonium was first produced and isolated on February 23, 1941 by Dr. Glenn T. Seaborg, McMillan, J. W. Kennedy, and A. C. Wahl by deuteron bombardment of uranium in the 60-inch cyclotron at Berkeley. The discovery was kept secret due to the war. It was named after the planet Pluto, having been discovered directly after neptunium (which itself was one higher on the periodic table than uranium), by analogy with the ordering of the planets in the solar system. Seaborg chose the letters "Pu" as a joke, which passed without notice into the periodic table. During the Manhattan Project, large reactors were set up in Hanford, Washington for the production of plutonium, which was used in two of the first atomic bombs (the first was tested at Trinity site, the second dropped on Hiroshima, Japan the third was dropped on Nagasaki).
Large stockpiles of plutonium were built up by both the old Soviet Union and the United States during the Cold War—it was estimated that 300,000 kg of plutonium had been accumulated by 1982. Since the end of the Cold War, these stockpiles have become a focus of nuclear proliferation concerns. In 2002, the United States Department of Energy took possession of 34 metric tons of excess weapons grade plutonium stockpiles from the United States Department of Defense, and as of early 2003 was considering converting several nuclear power plants in the US from enriched uranium fuel to MOX fuel as a means of disposing of these.
During the initial years after the discovery of plutonium, when its biological and physical properties were very poorly understood, a series of human radiation experiments were performed by the U.S. government and by private organizations acting on its behalf. During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects. In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition. The injections were made without the informed consent of those patients. [1]
The episode is now considered to be a serious breach of medical ethics and of the Hippocratic Oath, and has been sharply criticised as failing "both the test of our national values and the test of humanity." [2]
Occurrence
While almost all plutonium is manufactured synthetically, extremely tiny trace amounts are found naturally in uranium ores. These come about by a process of neutron capture by 238U nuclei, initially forming 239U; two subsequent beta decays then form 239Pu (with a 239Np intermediary), which has a half-life of 24,100 years. This is also the process used to manufacture 239Pu in nuclear reactors. Some traces of 244Pu remain from the birth of the solar system from waste of supernovae, because its half-life (80 million yrs) is fairly long.
A relatively high concentration of plutonium was discovered at the Natural nuclear fission reactor in Oklo, Gabon in 1972. Since 1945, about 10 tons of plutonium have been released onto Earth through nuclear explosions.
Manufacture
Pu-239
Plutonium-239 is one of the two fissile materials used for the production of nuclear weapons and in some nuclear reactors as a source of energy. The other fissile material is uranium-235. Plutonium-239 is virtually nonexistent in nature. It is made by bombarding uranium-238 with neutrons in a nuclear reactor. Uranium-238 is present in quantity in most reactor fuel; hence plutonium-239 is continuously made in these reactors. Since plutonium-239 can itself be split by neutrons to release energy, plutonium-239 provides a portion of the energy generation in a nuclear reactor.
Pu-238
There are small amounts of Pu-238 in the plutonium of usual plutonium-producing reactors. However, isotopic separation would be quite expensive compared to another method: When an U-235 atom captures a neutron, it is converted to an excited state of U-236. Some of the excited U-236 nuclei undergo fission, but some decay to the ground state of U-236 by emitting gamma radiation. Further neutron capture creates U-237 which has a half-life of 7 days and thus quickly decays to Np-237. Since nearly all neptunium is produced in this way or consists of isotopes which decay quickly, one gets nearly pure Np-237 by chemical separation of neptunium. After this chemical separation, Np-237 is again irradiated by reactor neutrons to be converted to Np-238 which decays to Pu-238 with a half-life of 2 days.
Compounds
Plutonium reacts readily with oxygen, forming PuO and PuO2, as well as intermediate oxides. It reacts with the halides, giving rise to compounds such as PuX3 where X can be F, Cl, Br or I; PuF4 is also seen. The following oxyhalides are observed: PuOCl, PuOBr and PuOI. It will react with carbon to form PuC, nitrogen to form PuN and silicon to form PuSi2.
Plutonium like other actinides readily forms a dioxide plutonyl core (PuO2). In the environment, this plutonyl core readily complexes with carbonate as well as other oxygen moeities (OH-, NO2-, NO3-, and SO4-2) to form charged complexes which can be readily mobile with low affinities to soil.
- PuO2(CO3)1-2
- PuO2(CO3)2-4
- PuO2(CO3)3-6
PuO2 formed from neutralizing highly acidic nitric acid solutions tends to form polymeric PuO2 which is resistant to complexation. Plutonium also readily shifts valences between the +3, +4, +5 and +6 states. It is common for some fraction of plutonium in solution to exist in all of these states in equilibrium.
Allotropes
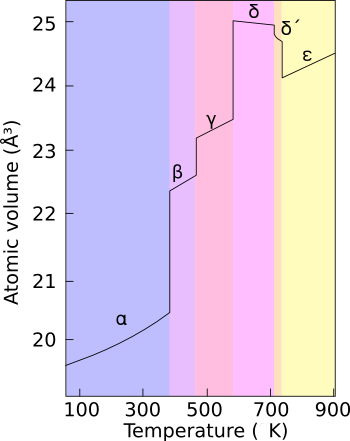
Even at ambient pressure, plutonium occurs in a variety of allotropes. These allotropes differ widely in crystal structure and density; the α and δ allotropes differ in density by more than 25% at the same volume.
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. The reasons for the complicated phase diagram are not entirely understood; recent research has focused on constructing accurate computer models of the phase transitions.Template:Inote
In weapons applications, plutonium is often alloyed with another metal (e.g., delta phase with a small percentage of gallium) to increase phase stability and thereby enhance workability and ease of handling. Interestingly, in fission weapons, the explosive shock waves used to compress a plutonium core will also cause a transition from the usual delta phase plutonium to the denser alpha phase, significantly helping to achieve supercriticality.
Isotopes
Twenty-one plutonium radioisotopes have been characterized. The most stable are Pu-244, with a half-life of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none are very stable (all have half-lives less than one second).
The isotopes of plutonium range in atomic weight from 228.0387 u (Pu-228) to 247.074 u (Pu-247). The primary decay modes before the most stable isotope, Pu-244, are spontaneous fission and alpha emission; the primary mode after is beta emission. The primary decay products before Pu-244 are uranium and neptunium isotopes (neglecting the wide range of daughter nuclei created by fission processes), and the primary products after are americium isotopes.
Key isotopes for applications are Pu-239, which is suitable for use in nuclear weapons and nuclear reactors, and Pu-238, which is suitable for use in radioisotope thermoelectric generators; see above for more details. The isotope Pu-240 undergoes spontaneous fission very readily, and is produced when Pu-239 is exposed to neutrons. The presence of Pu-240 in a material limits its nuclear bomb potential since it emits neutrons randomly, increasing the difficulty of initiating accurately the chain reaction at the desired instant and thus reducing the bomb's reliability and power. Plutonium consisting of more than about 90% Pu-239 is called weapon-grade plutonium; plutonium obtained from commercial reactors generally contains at least 20% Pu-240 and is called reactor-grade plutonium.
Precautions
All isotopes and compounds of plutonium are toxic and radioactive. While plutonium is sometimes described in media reports as "the most toxic substance known to man", there is general agreement among experts in the field that this is incorrect. As of 2003, there has yet to be a single human death officially attributed to exposure to plutonium itself (with the exception of plutonium-related criticality accidents). Naturally-occurring radium is about 200 times more radiotoxic than plutonium, and some organic toxins like Botulin toxin are still more toxic. Botulin toxin, in particular, has a lethal dose of 300pg/kg, far less than the quantity of plutonium that poses a significant cancer risk. In addition, beta and gamma emitters (including the C-14 and K-40 in nearly all food) can cause cancer on casual contact, which alpha emitters cannot.
Orally, plutonium is less toxic (non-oncogenically speaking) than several common substances, including caffeine, acetaminophen, some vitamins, pseudoephedrine, and any number of plants and fungi. It is perhaps somewhat more toxic than pure ethanol, but less so than tobacco and many illegal drugs (some such as marijuana are negligibly toxic). From a purely chemical standpoint, its toxicity is probably on par with lead and other heavy metals.
That said, there is no doubt that plutonium may be extremely dangerous when handled incorrectly. The alpha radiation it emits does not penetrate the skin, but can irradiate internal organs when plutonium is inhaled or ingested. Particularly at risk are the skeleton, whose surface it is likely to be absorbed on, and the liver, where it will collect and become concentrated. Extremely fine particles of plutonium (on the order of micrograms) can cause lung cancer if inhaled.
Other substances including ricin, botulinum toxin and tetanus toxin are fatal in doses of (sometimes far) under one milligram, and others (the nerve agents, the amanita toxin, the fugu toxin) are in the range of a few milligrams. As such, plutonium is not unusual in terms of toxicity, even by inhalation. In addition, those substances are fatal in hours to days, whereas plutonium (and other cancer-causing radioactives) give an increased chance of illness decades in the future. Considerably larger amounts may cause acute radiation poisoning and death if ingested or inhaled; however, so far, no human is known to have immediately died because of inhaling or ingesting plutonium and many people have measurable amounts of plutonium in their bodies.
It must be noted, however, that in contrast to naturally occurring radioisotopes such as radium or C-14, plutonium was manufactured, concentrated, and isolated in large amounts (hundreds of metric tons) during the Cold War for weapons production. These piles, whether in weapons form or otherwise, could pose a significant toxicologic risk, largely because, unlike chemical or biological agents, there is no practical way to destroy them.
Toxicity issues aside, care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass. Despite not being confined by external pressure as is required for a nuclear weapon, it will nevertheless heat itself and break whatever confining environment it is in. Shape is relevant; compact shapes such as spheres are to be avoided. Plutonium in solution is more likely to form a critical mass than the solid form. A weapon-scale nuclear explosion cannot occur accidentally, since it requires a greatly supercritical mass in order to explode rather than simply melt or fragment. However, a marginally critical mass will cause a lethal dose of radiation and has in fact done so in the past on several occasions.
Multiple criticality accidents have occurred in the past, some of them with lethal consequences. Careless handling of tungsten carbide bricks around a 6.2 kg plutonium sphere resulted in a lethal dose of radiation at Los Alamos on August 21, 1945, when scientist Harry Daghlian received a dose estimated to be 510 rems (5.1 Sv) and died four weeks later. Nine months later, another Los Alamos scientist, Louis Slotin, died from a similar accident involving a beryllium reflector and the exact same plutonium core (the so-called “demon core”) that had previously claimed the life of Daghlian. These incidents were fairly accurately portrayed in the 1989 film Fat Man and Little Boy. In 1958, during a process of purifying plutonium at Los Alamos, a critical mass was formed in a mixing vessel, which resulted in the death of a crane operator. Other accidents of this sort have occurred in the Soviet Union, Japan, and many other countries. (See List of nuclear accidents)
Metallic plutonium is also a fire hazard, especially if the material is finely divided. It reacts chemically with oxygen and water which may result in an accumulation of plutonium hydride, a pyrophoric substance; that is, a material that will ignite in air at room temperature. Plutonium expands considerably in size as it oxidizes and thus may break its container. The radioactivity of the burning material is an additional hazard. Magnesium oxide sand is the most effective material for extinguishing a plutonium fire. It cools the burning material, acting as a heat sink, and also blocks off oxygen. Water is also effective. There was a major plutonium-initiated fire at the Rocky Flats Plant near Boulder, Colorado in 1969 [3]. To avoid these problems, special precautions are necessary to store or handle plutonium in any form; generally a dry inert atmosphere is required [4].
References
- ^ Crooks, William J. (2006-02-15). "Nuclear Criticality Safety Engineering Training Module 10 - Criticality Safety in Material Processing Operations, Part 1" (PDF).
{{cite web}}: Unknown parameter|publishyear=ignored (help)
- Los Alamos National Laboratory - Plutonium
- It's Elemental - Plutonium
- DOE Plutonium fact sheet (PDF)
- End of the Plutonium Age, D. Samuels, Discover Magazine, vol. 26, no. 11 (November, 2005).
- WebElements.com - Plutonium
- EnvironmentalChemistry.com - Plutonium (JavaScript required)
- Federation of American Scientists - Plutonium production
- nuclearweaponarchive.org - Plutonium Manufacture and Fabrication
- Ambient pressure phase diagram of plutonium - A unified theory for α-Pu and δ-Pu, P. Söderlind, Europhys. Lett., 55 (4), p. 525 (2001).
External links
- "A Perspective on the Dangers of Plutonium" by Lawrence Livermore National Laboratory
- collection of articles on plutonium at the Canadian Coalition for Nuclear Responsibility
- The Myth of Plutonium Toxicity
- Criticality Accidents Report Issued
- Nuclear Accidents Timeline
- Nuclear Weapons: Disposal Options for Surplus Weapons-Usable Plutonium
- Unraveling the Phase Diagram of Plutonium
- Physical, Nuclear, and Chemical, Properties of Plutonium from IEER
- Nuclear Files.org Information regarding world plutonium inventories
