Stroke: Difference between revisions
→Subtypes: wikify a few things including walking gait |
→Subtypes: wikify |
||
| Line 60: | Line 60: | ||
* numbness |
* numbness |
||
* reduction in sensory or vibratory sensation |
* reduction in sensory or vibratory sensation |
||
* initial flaccidity (hypotonicity), replaced by spasticity (hypertonicity), hyperreflexia, and obligatory synergies.<ref name=OSul07_719>{{cite book | last1 = O'Sullivan | first1 = Susan.B | title = Physical Rehabilitation | chapter = Stroke | volume = 5 | editors = O'Sullivan, S.B., and Schmitz, T.J. | publisher = F.A. Davis Company | year = 2007 | location = Philadelphia | page = 719 |ref=harv}}</ref> |
* initial [[flaccidity]] (hypotonicity), replaced by [[spasticity]] (hypertonicity), [[hyperreflexia]], and obligatory synergies.<ref name=OSul07_719>{{cite book | last1 = O'Sullivan | first1 = Susan.B | title = Physical Rehabilitation | chapter = Stroke | volume = 5 | editors = O'Sullivan, S.B., and Schmitz, T.J. | publisher = F.A. Davis Company | year = 2007 | location = Philadelphia | page = 719 |ref=harv}}</ref> |
||
In most cases, the symptoms affect only one side of the body ([[unilateral]]). Depending on the part of the brain affected, the defect in the brain is ''usually'' on the opposite side of the body. However, |
In most cases, the symptoms affect only one side of the body ([[unilateral]]). Depending on the part of the brain affected, the defect in the brain is ''usually'' on the opposite side of the body. However, since these pathways also travel in the [[spinal cord]] and any lesion there can also produce these symptoms, the presence of any one of these symptoms does not necessarily indicate a stroke. |
||
In addition to the above CNS pathways, the ''[[brainstem]]'' gives rise to most of the twelve [[cranial nerves]]. A stroke affecting the brain stem and brain therefore can produce symptoms relating to deficits in these cranial nerves: |
In addition to the above CNS pathways, the ''[[brainstem]]'' gives rise to most of the twelve [[cranial nerves]]. A stroke affecting the brain stem and brain therefore can produce symptoms relating to deficits in these cranial nerves: |
||
Revision as of 10:02, 10 May 2012
| Stroke | |
|---|---|
| Specialty | Neurology, neurosurgery |
| Frequency | 0.24% (France), 0.6% (Novosibirsk) |
A stroke, or cerebrovascular accident (CVA), is the rapid loss of brain function(s) due to disturbance in the blood supply to the brain. This can be due to ischemia (lack of blood flow) caused by blockage (thrombosis, arterial embolism), or a hemorrhage (leakage of blood).[1] As a result, the affected area of the brain cannot function, which might result in an inability to move one or more limbs on one side of the body, inability to understand or formulate speech, or an inability to see one side of the visual field.[2]
A stroke is a medical emergency and can cause permanent neurological damage, complications, and death. It is the leading cause of adult disability in the United States and Europe and the second leading cause of death worldwide.[citation needed] Risk factors for stroke include old age, hypertension (high blood pressure), previous stroke or transient ischemic attack (TIA), diabetes, high cholesterol, cigarette smoking and atrial fibrillation.[2] High blood pressure is the most important modifiable risk factor of stroke.[2]
An ischemic stroke is occasionally treated in a hospital with thrombolysis (also known as a "clot buster"), and some hemorrhagic strokes benefit from neurosurgery. Treatment to recover any lost function is termed stroke rehabilitation, ideally in a stroke unit and involving health professions such as speech and language therapy, physical therapy and occupational therapy. Prevention of recurrence may involve the administration of antiplatelet drugs such as aspirin and dipyridamole, control and reduction of hypertension, and the use of statins. Selected patients may benefit from carotid endarterectomy and the use of anticoagulants.[2]
Classification
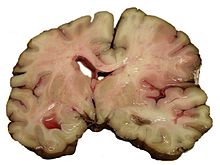
Strokes can be classified into two major categories: ischemic and hemorrhagic.[3] Ischemic strokes are those that are caused by interruption of the blood supply, while hemorrhagic strokes are the ones which result from rupture of a blood vessel or an abnormal vascular structure. About 87% of strokes are caused by ischemia, and the remainder by hemorrhage. Some hemorrhages develop inside areas of ischemia ("hemorrhagic transformation"). It is unknown how many hemorrhages actually start as ischemic stroke.[2]
Ischemic
In an ischemic stroke, blood supply to part of the brain is decreased, leading to dysfunction of the brain tissue in that area. There are four reasons why this might happen:
- Thrombosis (obstruction of a blood vessel by a blood clot forming locally)
- Embolism (obstruction due to an embolus from elsewhere in the body, see below),[2]
- Systemic hypoperfusion (general decrease in blood supply, e.g., in shock)[4]
- Venous thrombosis.[5]
Stroke without an obvious explanation is termed "cryptogenic" (of unknown origin); this constitutes 30-40% of all ischemic strokes.[2][6]
There are various classification systems for acute ischemic stroke. The Oxford Community Stroke Project classification (OCSP, also known as the Bamford or Oxford classification) relies primarily on the initial symptoms; based on the extent of the symptoms, the stroke episode is classified as total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), lacunar infarct (LACI) or posterior circulation infarct (POCI). These four entities predict the extent of the stroke, the area of the brain affected, the underlying cause, and the prognosis.[7][8] The TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification is based on clinical symptoms as well as results of further investigations; on this basis, a stroke is classified as being due to (1) thrombosis or embolism due to atherosclerosis of a large artery, (2) embolism of cardiac origin, (3) occlusion of a small blood vessel, (4) other determined cause, (5) undetermined cause (two possible causes, no cause identified, or incomplete investigation).[2][9]
Hemorrhagic
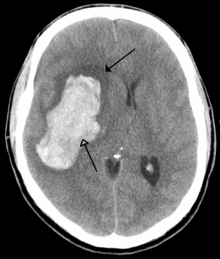
Intracranial hemorrhage is the accumulation of blood anywhere within the skull vault. A distinction is made between intra-axial hemorrhage (blood inside the brain) and extra-axial hemorrhage (blood inside the skull but outside the brain). Intra-axial hemorrhage is due to intraparenchymal hemorrhage or intraventricular hemorrhage (blood in the ventricular system). The main types of extra-axial hemorrhage are epidural hematoma (bleeding between the dura mater and the skull), subdural hematoma (in the subdural space) and subarachnoid hemorrhage (between the arachnoid mater and pia mater). Most of the hemorrhagic stroke syndromes have specific symptoms (e.g., headache, previous head injury).
Signs and symptoms
Stroke symptoms typically start suddenly, over seconds to minutes, and in most cases do not progress further. The symptoms depend on the area of the brain affected. The more extensive the area of brain affected, the more functions that are likely to be lost. Some forms of stroke can cause additional symptoms. For example, in intracranial hemorrhage, the affected area may compress other structures. Most forms of stroke are not associated with headache, apart from subarachnoid hemorrhage and cerebral venous thrombosis and occasionally intracerebral hemorrhage.
Early recognition
Various systems have been proposed to increase recognition of stroke by patients, relatives and emergency first responders. A systematic review, updating a previous systematic review from 1994, looked at a number of trials to evaluate how well different physical examination findings are able to predict the presence or absence of stroke. It was found that sudden-onset face weakness, arm drift (i.e., if a person, when asked to raise both arms, involuntarily lets one arm drift downward) and abnormal speech are the findings most likely to lead to the correct identification of a case of stroke (+ likelihood ratio of 5.5 when at least one of these is present). Similarly, when all three of these are absent, the likelihood of stroke is significantly decreased (– likelihood ratio of 0.39).[10] While these findings are not perfect for diagnosing stroke, the fact that they can be evaluated relatively rapidly and easily make them very valuable in the acute setting.
Proposed systems include FAST (stroke) (face, arm, speech, and time),[11] as advocated by the Department of Health (United Kingdom) and The Stroke Association, the American Stroke Association (www.strokeassociation.org), National Stroke Association (US www.stroke.org), the Los Angeles Prehospital Stroke Screen (LAPSS)[12] and the Cincinnati Prehospital Stroke Scale (CPSS).[13] Use of these scales is recommended by professional guidelines.[14]
For people referred to the emergency room, early recognition of stroke is deemed important as this can expedite diagnostic tests and treatments. A scoring system called ROSIER (recognition of stroke in the emergency room) is recommended for this purpose; it is based on features from the medical history and physical examination.[14][15]
Subtypes
If the area of the brain affected contains one of the three prominent central nervous system pathways—the spinothalamic tract, corticospinal tract, and dorsal column (medial lemniscus), symptoms may include:
- hemiplegia and muscle weakness of the face
- numbness
- reduction in sensory or vibratory sensation
- initial flaccidity (hypotonicity), replaced by spasticity (hypertonicity), hyperreflexia, and obligatory synergies.[16]
In most cases, the symptoms affect only one side of the body (unilateral). Depending on the part of the brain affected, the defect in the brain is usually on the opposite side of the body. However, since these pathways also travel in the spinal cord and any lesion there can also produce these symptoms, the presence of any one of these symptoms does not necessarily indicate a stroke.
In addition to the above CNS pathways, the brainstem gives rise to most of the twelve cranial nerves. A stroke affecting the brain stem and brain therefore can produce symptoms relating to deficits in these cranial nerves:
- altered smell, taste, hearing, or vision (total or partial)
- drooping of eyelid (ptosis) and weakness of ocular muscles
- decreased reflexes: gag, swallow, pupil reactivity to light
- decreased sensation and muscle weakness of the face
- balance problems and nystagmus
- altered breathing and heart rate
- weakness in sternocleidomastoid muscle with inability to turn head to one side
- weakness in tongue (inability to protrude and/or move from side to side)
If the cerebral cortex is involved, the CNS pathways can again be affected, but also can produce the following symptoms:
- aphasia (difficulty with verbal expression, auditory comprehension, reading and/or writing Broca's or Wernicke's area typically involved)
- dysarthria (motor speech disorder resulting from neurological injury)
- apraxia (altered voluntary movements)
- visual field defect
- memory deficits (involvement of temporal lobe)
- hemineglect (involvement of parietal lobe)
- disorganized thinking, confusion, hypersexual gestures (with involvement of frontal lobe)
- lack of insight of his or her, usually stroke-related, disability
If the cerebellum is involved, the patient may have the following:
- altered walking gait
- altered movement coordination
- vertigo and or disequilibrium
Associated symptoms
Loss of consciousness, headache, and vomiting usually occurs more often in hemorrhagic stroke than in thrombosis because of the increased intracranial pressure from the leaking blood compressing the brain.
If symptoms are maximal at onset, the cause is more likely to be a subarachnoid hemorrhage or an embolic stroke.
Causes
This section needs additional citations for verification. (September 2008) |
Thrombotic stroke
In thrombotic stroke a thrombus[17] (blood clot) usually forms around atherosclerotic plaques. Since blockage of the artery is gradual, onset of symptomatic thrombotic strokes is slower. A thrombus itself (even if non-occluding) can lead to an embolic stroke (see below) if the thrombus breaks off, at which point it is called an "embolus." Two types of thrombosis can cause stroke:
- Large vessel disease involves the common and internal carotids, vertebral, and the Circle of Willis.[18] Diseases that may form thrombi in the large vessels include (in descending incidence): atherosclerosis, vasoconstriction (tightening of the artery), aortic, carotid or vertebral artery dissection, various inflammatory diseases of the blood vessel wall (Takayasu arteritis, giant cell arteritis, vasculitis), noninflammatory vasculopathy, Moyamoya disease and fibromuscular dysplasia.
- Small vessel disease involves the smaller arteries inside the brain: branches of the circle of Willis, middle cerebral artery, stem, and arteries arising from the distal vertebral and basilar artery.[19] Diseases that may form thrombi in the small vessels include (in descending incidence): lipohyalinosis (build-up of fatty hyaline matter in the blood vessel as a result of high blood pressure and aging) and fibrinoid degeneration[20] (stroke involving these vessels are known as lacunar infarcts) and microatheroma (small atherosclerotic plaques).[21]
Sickle-cell anemia,[22] which can cause blood cells to clump up and block blood vessels, can also lead to stroke. A stroke is the second leading killer of people under 20 who suffer from sickle-cell anemia.[23]
Embolic stroke
An embolic stroke refers to the blockage of an artery by an arterial embolus, a travelling particle or debris in the arterial bloodstream originating from elsewhere. An embolus is most frequently a thrombus, but it can also be a number of other substances including fat (e.g., from bone marrow in a broken bone), air, cancer cells or clumps of bacteria (usually from infectious endocarditis).
Because an embolus arises from elsewhere, local therapy solves the problem only temporarily. Thus, the source of the embolus must be identified. Because the embolic blockage is sudden in onset, symptoms usually are maximal at start. Also, symptoms may be transient as the embolus is partially resorbed and moves to a different location or dissipates altogether.
Emboli most commonly arise from the heart (especially in atrial fibrillation) but may originate from elsewhere in the arterial tree. In paradoxical embolism, a deep vein thrombosis embolises through an atrial or ventricular septal defect in the heart into the brain.
Cardiac causes can be distinguished between high and low-risk:[24]
- High risk: atrial fibrillation and paroxysmal atrial fibrillation, rheumatic disease of the mitral or aortic valve disease, artificial heart valves, known cardiac thrombus of the atrium or ventricle, sick sinus syndrome, sustained atrial flutter, recent myocardial infarction, chronic myocardial infarction together with ejection fraction <28 percent, symptomatic congestive heart failure with ejection fraction <30 percent, dilated cardiomyopathy, Libman-Sacks endocarditis, Marantic endocarditis, infective endocarditis, papillary fibroelastoma, left atrial myxoma and coronary artery bypass graft (CABG) surgery.
- Low risk/potential: calcification of the annulus (ring) of the mitral valve, patent foramen ovale (PFO), atrial septal aneurysm, atrial septal aneurysm with patent foramen ovale, left ventricular aneurysm without thrombus, isolated left atrial "smoke" on echocardiography (no mitral stenosis or atrial fibrillation), complex atheroma in the ascending aorta or proximal arch.
Systemic hypoperfusion
Systemic hypoperfusion is the reduction of blood flow to all parts of the body. It is most commonly due to cardiac pump failure from cardiac arrest or arrhythmias, or from reduced cardiac output as a result of myocardial infarction, pulmonary embolism, pericardial effusion, or bleeding. Hypoxemia (low blood oxygen content) may precipitate the hypoperfusion. Because the reduction in blood flow is global, all parts of the brain may be affected, especially "watershed" areas - border zone regions supplied by the major cerebral arteries. A watershed stroke refers to the condition when blood supply to these areas is compromised. Blood flow to these areas does not necessarily stop, but instead it may lessen to the point where brain damage can occur. This phenomenon is also referred to as "last meadow" to point to the fact that in irrigation the last meadow receives the least amount of water.
Venous thrombosis
Cerebral venous sinus thrombosis leads to stroke due to locally increased venous pressure, which exceeds the pressure generated by the arteries. Infarcts are more likely to undergo hemorrhagic transformation (leaking of blood into the damaged area) than other types of ischemic stroke.[5]
Intracerebral hemorrhage
It generally occurs in small arteries or arterioles and is commonly due to hypertension,[25] intracranial vascular malformations (including cavernous angiomas or arteriovenous malformations), cerebral amyloid angiopathy, or infarcts into which secondary haemorrhage has occurred.[2] Other potential causes are trauma, bleeding disorders, amyloid angiopathy, illicit drug use (e.g., amphetamines or cocaine). The hematoma enlarges until pressure from surrounding tissue limits its growth, or until it decompresses by emptying into the ventricular system, CSF or the pial surface. A third of intracerebral bleed is into the brain's ventricles. ICH has a mortality rate of 44 percent after 30 days, higher than ischemic stroke or subarachnoid hemorrhage (which technically may also be classified as a type of stroke[2]).
Silent stroke
A silent stroke is a stroke that does not have any outward symptoms, and the patients are typically unaware they have suffered a stroke. Despite not causing identifiable symptoms, a silent stroke still causes damage to the brain, and places the patient at increased risk for both transient ischemic attack and major stroke in the future. Conversely, those who have suffered a major stroke are at risk of having silent strokes.[26] In a broad study in 1998, more than 11 million people were estimated to have experienced a stroke in the United States. Approximately 770,000 of these strokes were symptomatic and 11 million were first-ever silent MRI infarcts or hemorrhages. Silent strokes typically cause lesions which are detected via the use of neuroimaging such as MRI. Silent strokes are estimated to occur at five times the rate of symptomatic strokes.[27][28] The risk of silent stroke increases with age, but may also affect younger adults and children, especially those with acute anemia.[27][29]
Pathophysiology
Ischemic
This section needs additional citations for verification. (September 2008) |
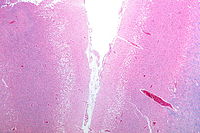

Ischemic stroke occurs because of a loss of blood supply to part of the brain, initiating the ischemic cascade.[30] Brain tissue ceases to function if deprived of oxygen for more than 60 to 90 seconds and after approximately three hours, will suffer irreversible injury possibly leading to death of the tissue, i.e., infarction. (This is why fibrinolytics such as alteplase are given only until three hours since the onset of the stroke.) Atherosclerosis may disrupt the blood supply by narrowing the lumen of blood vessels leading to a reduction of blood flow, by causing the formation of blood clots within the vessel, or by releasing showers of small emboli through the disintegration of atherosclerotic plaques.[31] Embolic infarction occurs when emboli formed elsewhere in the circulatory system, typically in the heart as a consequence of atrial fibrillation, or in the carotid arteries, break off, enter the cerebral circulation, then lodge in and occlude brain blood vessels. Since blood vessels in the brain are now occluded, the brain becomes low in energy, and thus it resorts into using anaerobic respiration within the region of brain tissue affected by ischemia. Unfortunately, this kind of respiration produces less adenosine triphosphate (ATP) but releases a by-product called lactic acid. Lactic acid is an irritant which could potentially destroy cells since it is an acid and disrupts the normal acid-base balance in the brain. The ischemia area is referred to as the "ischemic penumbra".[32]
Then, as oxygen or glucose becomes depleted in ischemic brain tissue, the production of high energy phosphate compounds such as adenosine triphosphate (ATP) fails, leading to failure of energy-dependent processes (such as ion pumping) necessary for tissue cell survival. This sets off a series of interrelated events that result in cellular injury and death. A major cause of neuronal injury is release of the excitatory neurotransmitter glutamate. The concentration of glutamate outside the cells of the nervous system is normally kept low by so-called uptake carriers, which are powered by the concentration gradients of ions (mainly Na+) across the cell membrane. However, stroke cuts off the supply of oxygen and glucose which powers the ion pumps maintaining these gradients. As a result the transmembrane ion gradients run down, and glutamate transporters reverse their direction, releasing glutamate into the extracellular space. Glutamate acts on receptors in nerve cells (especially NMDA receptors), producing an influx of calcium which activates enzymes that digest the cells' proteins, lipids and nuclear material. Calcium influx can also lead to the failure of mitochondria, which can lead further toward energy depletion and may trigger cell death due to apoptosis.
Ischemia also induces production of oxygen free radicals and other reactive oxygen species. These react with and damage a number of cellular and extracellular elements. Damage to the blood vessel lining or endothelium is particularly important. In fact, many antioxidant neuroprotectants such as uric acid and NXY-059 work at the level of the endothelium and not in the brain per se. Free radicals also directly initiate elements of the apoptosis cascade by means of redox signaling.[23]
These processes are the same for any type of ischemic tissue and are referred to collectively as the ischemic cascade. However, brain tissue is especially vulnerable to ischemia since it has little respiratory reserve and is completely dependent on aerobic metabolism, unlike most other organs.
Brain tissue survival can be improved to some extent if one or more of these processes is inhibited. Drugs that scavenge reactive oxygen species, inhibit apoptosis, or inhibit excitatory neurotransmitters, for example, have been shown experimentally to reduce tissue injury caused by ischemia. Agents that work in this way are referred to as being neuroprotective. Until recently, human clinical trials with neuroprotective agents have failed, with the probable exception of deep barbiturate coma. However, more recently NXY-059, the disulfonyl derivative of the radical-scavenging spintrap phenylbutylnitrone, is reported to be neuroprotective in stroke.[33] This agent appears to work at the level of the blood vessel lining or endothelium. Unfortunately, after producing favorable results in one large-scale clinical trial, a second trial failed to show favorable results.[23]
In addition to injurious effects on brain cells, ischemia and infarction can result in loss of structural integrity of brain tissue and blood vessels, partly through the release of matrix metalloproteases, which are zinc- and calcium-dependent enzymes that break down collagen, hyaluronic acid, and other elements of connective tissue. Other proteases also contribute to this process. The loss of vascular structural integrity results in a breakdown of the protective blood brain barrier that contributes to cerebral edema, which can cause secondary progression of the brain injury.
As is the case with any type of brain injury, the immune system is activated by cerebral infarction and may under some circumstances exacerbate the injury caused by the infarction. Inhibition of the inflammatory response has been shown experimentally to reduce tissue injury due to cerebral infarction, but this has not proved out in clinical studies.
Hemorrhagic
Hemorrhagic strokes result in tissue injury by causing compression of tissue from an expanding hematoma or hematomas. This can distort and injure tissue. In addition, the pressure may lead to a loss of blood supply to affected tissue with resulting infarction, and the blood released by brain hemorrhage appears to have direct toxic effects on brain tissue and vasculature.[23][34] Inflammation contributes to the secondary brain injury after hemorrhage.[34]
Diagnosis

Stroke is diagnosed through several techniques: a neurological examination (such as the Nihss), CT scans (most often without contrast enhancements) or MRI scans, Doppler ultrasound, and arteriography. The diagnosis of stroke itself is clinical, with assistance from the imaging techniques. Imaging techniques also assist in determining the subtypes and cause of stroke. There is yet no commonly used blood test for the stroke diagnosis itself, though blood tests may be of help in finding out the likely cause of stroke.[35]
Definition
The traditional definition of stroke, devised by the World Health Organization in the 1970s,[36] is a "neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours". This definition was supposed to reflect the reversibility of tissue damage and was devised for the purpose, with the time frame of 24 hours being chosen arbitrarily. The 24-hour limit divides stroke from transient ischemic attack, which is a related syndrome of stroke symptoms that resolve completely within 24 hours.[2] With the availability of treatments that, when given early, can reduce stroke severity, many now prefer alternative concepts, such as brain attack and acute ischemic cerebrovascular syndrome (modeled after heart attack and acute coronary syndrome, respectively), that reflect the urgency of stroke symptoms and the need to act swiftly.[37]
Physical examination
A physical examination, including taking a medical history of the symptoms and a neurological status, helps giving an evaluation of the location and severity of a stroke. It can give a standard score on e.g., the NIH stroke scale.
Imaging
For diagnosing ischemic stroke in the emergency setting:[38]
- CT scans (without contrast enhancements)
- sensitivity= 16%
- specificity= 96%
- MRI scan
- sensitivity= 83%
- specificity= 98%
For diagnosing hemorrhagic stroke in the emergency setting:
- CT scans (without contrast enhancements)
- sensitivity= 89%
- specificity= 100%
- MRI scan
- sensitivity= 81%
- specificity= 100%
For detecting chronic hemorrhages, MRI scan is more sensitive.[39]
For the assessment of stable stroke, nuclear medicine scans SPECT and PET/CT may be helpful. SPECT documents cerebral blood flow and PET with FDG isotope the metabolic activity of the neurons.
Underlying cause
When a stroke has been diagnosed, various other studies may be performed to determine the underlying cause. With the current treatment and diagnosis options available, it is of particular importance to determine whether there is a peripheral source of emboli. Test selection may vary, since the cause of stroke varies with age, comorbidity and the clinical presentation. Commonly used techniques include:
- an ultrasound/doppler study of the carotid arteries (to detect carotid stenosis) or dissection of the precerebral arteries;
- an electrocardiogram (ECG) and echocardiogram (to identify arrhythmias and resultant clots in the heart which may spread to the brain vessels through the bloodstream);
- a Holter monitor study to identify intermittent arrhythmias;
- an angiogram of the cerebral vasculature (if a bleed is thought to have originated from an aneurysm or arteriovenous malformation);
- blood tests to determine hypercholesterolemia, bleeding diathesis and some rarer causes such as homocysteinuria.
Prevention
Given the disease burden of strokes, prevention is an important public health concern.[40] Primary prevention is less effective than secondary prevention (as judged by the number needed to treat to prevent one stroke per year).[40] Recent guidelines detail the evidence for primary prevention in stroke.[41] Because stroke may indicate underlying atherosclerosis, it is important to determine the patient's risk for other cardiovascular diseases such as coronary heart disease. Conversely, aspirin confers some protection against first stroke in patients who have suffered a myocardial infarction or patients with a high cardiovascular risk.[42][43]
Risk factors
The most important modifiable risk factors for stroke are high blood pressure and atrial fibrillation (although magnitude of this effect is small: the evidence from the Medical Research Council trials is that 833 patients have to be treated for 1 year to prevent one stroke[44][45]). Other modifiable risk factors include high blood cholesterol levels, diabetes, cigarette smoking[46][47] (active and passive), heavy alcohol consumption[48] and drug use,[49] lack of physical activity, obesity, processed red meat consumption[50] and unhealthy diet.[51] Alcohol use could predispose to ischemic stroke, and intracerebral and subarachnoid hemorrhage via multiple mechanisms (for example via hypertension, atrial fibrillation, rebound thrombocytosis and platelet aggregation and clotting disturbances).[52] The drugs most commonly associated with stroke are cocaine, amphetamines causing hemorrhagic stroke, but also over-the-counter cough and cold drugs containing sympathomimetics.[53][54]
No high quality studies have shown the effectiveness of interventions aimed at weight reduction, promotion of regular exercise, reducing alcohol consumption or smoking cessation.[55] Nonetheless, given the large body of circumstantial evidence, best medical management for stroke includes advice on diet, exercise, smoking and alcohol use.[56] Medication or drug therapy is the most common method of stroke prevention; carotid endarterectomy can be a useful surgical method of preventing stroke.
Blood pressure
Hypertension accounts for 35-50% of stroke risk.[57] Epidemiological studies suggest that even a small blood pressure reduction (5 to 6 mmHg systolic, 2 to 3 mmHg diastolic) would result in 40% fewer strokes.[58] Lowering blood pressure has been conclusively shown to prevent both ischemic and hemorrhagic strokes.[59][60] It is equally important in secondary prevention.[61] Even patients older than 80 years and those with isolated systolic hypertension benefit from antihypertensive therapy.[62][63][64] Studies show that intensive antihypertensive therapy results in a greater risk reduction.[65] The available evidence does not show large differences in stroke prevention between antihypertensive drugs —therefore, other factors such as protection against other forms of cardiovascular disease should be considered and cost.[65][66]
Atrial fibrillation
Patients with atrial fibrillation have a risk of 5% each year to develop stroke, and this risk is even higher in those with valvular atrial fibrillation.[67] Depending on the stroke risk, anticoagulation with medications such as coumarins or aspirin is warranted for stroke prevention.[68]
Blood lipids
High cholesterol levels have been inconsistently associated with (ischemic) stroke.[60][69] Statins have been shown to reduce the risk of stroke by about 15%.[70] Since earlier meta-analyses of other lipid-lowering drugs did not show a decreased risk,[71] statins might exert their effect through mechanisms other than their lipid-lowering effects.[70]
Diabetes mellitus
Patients with diabetes mellitus are 2 to 3 times more likely to develop stroke, and they commonly have hypertension and hyperlipidemia. Intensive disease control has been shown to reduce microvascular complications such as nephropathy and retinopathy but not macrovascular complications such as stroke.[72][73]
Anticoagulation drugs
Oral anticoagulants such as warfarin have been the mainstay of stroke prevention for over 50 years. However, several studies have shown that aspirin and antiplatelet drugs are highly effective in secondary prevention after a stroke or transient ischemic attack.[42] Low doses of aspirin (for example 75–150 mg) are as effective as high doses but have fewer side effects; the lowest effective dose remains unknown.[74] Thienopyridines (clopidogrel, ticlopidine) "might be slightly more effective" than aspirin and have a decreased risk of gastrointestinal bleeding, but they are more expensive.[75] Their exact role remains controversial. Ticlopidine has more skin rash, diarrhea, neutropenia and thrombotic thrombocytopenic purpura.[75] Dipyridamole can be added to aspirin therapy to provide a small additional benefit, even though headache is a common side effect.[76] Low-dose aspirin is also effective for stroke prevention after sustaining a myocardial infarction.[43] Except for in atrial fibrillation, oral anticoagulants are not advised for stroke prevention —any benefit is offset by bleeding risk.[77]
In primary prevention however, antiplatelet drugs did not reduce the risk of ischemic stroke while increasing the risk of major bleeding.[78][79] Further studies are needed to investigate a possible protective effect of aspirin against ischemic stroke in women.[80][81]
Surgery
Surgical procedures such as carotid endarterectomy or carotid angioplasty can be used to remove significant atherosclerotic narrowing (stenosis) of the carotid artery, which supplies blood to the brain. There is a large body of evidence supporting this procedure in selected cases.[56] Endarterectomy for a significant stenosis has been shown to be useful in the secondary prevention after a previous symptomatic stroke.[82] Carotid artery stenting has not been shown to be equally useful.[83][84] Patients are selected for surgery based on age, gender, degree of stenosis, time since symptoms and patients' preferences.[56] Surgery is most efficient when not delayed too long —the risk of recurrent stroke in a patient who has a 50% or greater stenosis is up to 20% after 5 years, but endarterectomy reduces this risk to around 5%. The number of procedures needed to cure one patient was 5 for early surgery (within two weeks after the initial stroke), but 125 if delayed longer than 12 weeks.[85][86]
Screening for carotid artery narrowing has not been shown to be a useful screening test in the general population.[87] Studies of surgical intervention for carotid artery stenosis without symptoms have shown only a small decrease in the risk of stroke.[88][89] To be beneficial, the complication rate of the surgery should be kept below 4%. Even then, for 100 surgeries, 5 patients will benefit by avoiding stroke, 3 will develop stroke despite surgery, 3 will develop stroke or die due to the surgery itself, and 89 will remain stroke-free but would also have done so without intervention.[56]
Nutritional and metabolic interventions
Nutrition, specifically the Mediterranean-style diet, has the potential for decreasing the risk of having a stroke by more than half.[90]
With regard to lowering homocysteine, a meta-analysis of previous trials has concluded that lowering homocysteine with folic acid and other supplements may reduce stroke risk.[91] However, the two largest randomized controlled trials included in the meta-analysis had conflicting results. One reported positive results;[92] whereas the other was negative.[93]
The European Society of Cardiology and the European Association for Cardiovascular Prevention and Rehabilitation have developed an interactive tool for prediction and managing the risk of heart attack and stroke in Europe. HeartScore is aimed at supporting clinicians in optimising individual cardiovascular risk reduction. The Heartscore Programme is available in 12 languages and offers web based or PC version.[94]
Management
Stroke unit
Ideally, people who have had a stroke are admitted to a "stroke unit", a ward or dedicated area in hospital staffed by nurses and therapists with experience in stroke treatment. It has been shown that people admitted to a stroke unit have a higher chance of surviving than those admitted elsewhere in hospital, even if they are being cared for by doctors without experience in stroke.[2]
When an acute stroke is suspected by history and physical examination, the goal of early assessment is to determine the cause. Treatment varies according to the underlying cause of the stroke, thromboembolic (ischemic) or hemorrhagic. A non-contrast head CT scan can rapidly identify a hemorrhagic stroke by imaging bleeding in or around the brain. If no bleeding is seen, a presumptive diagnosis of ischemic stroke is made.
Ischemic stroke
An ischemic stroke is caused by a thrombus (blood clot) occluding blood flow to an artery supplying the brain. Definitive therapy is aimed at removing the blockage by breaking the clot down (thrombolysis), or by removing it mechanically (thrombectomy). The more rapidly blood flow is restored to the brain, the fewer brain cells die.[95]
Other medical therapies are aimed at minimizing clot enlargement or preventing new clots from forming. To this end, treatment with medications such as aspirin, clopidogrel and dipyridamole may be given to prevent platelets from aggregating.[42]
Tight control of blood sugars in the first few hours does not improve outcomes and may cause harm.[96] High blood pressure is also not typically lowered as this has not been found to be helpful.
Thrombolysis
In an increasing number of primary stroke centers, pharmacologic thrombolysis ("clot busting") with the drug tissue plasminogen activator (tPA), is used to dissolve the clot and unblock the artery. However, the use of tPA in acute stroke is controversial. On one hand, it is endorsed by the American Heart Association and the American Academy of Neurology as the recommended treatment for acute stroke within three hours of onset of symptoms as long as there are not other contraindications (such as abnormal lab values, high blood pressure, or recent surgery). This position for tPA is based upon the findings of two studies by one group of investigators[97] which showed that tPA improves the chances for a good neurological outcome. When administered within the first three hours thrombolysis improves functional outcome without affecting mortality.[98] A recent study using alteplase for thrombolysis in ischemic stroke suggests clinical benefit with administration 3 to 4.5 hours after stroke onset.[99] However, in the NINDS trial 6.4% of patients with large strokes developed substantial brain hemorrhage as a complication from being given tPA. A recent study found the mortality to be higher among patients receiving tPA versus those who did not.[100] Additionally, it is the position of the American Academy of Emergency Medicine that objective evidence regarding the efficacy, safety, and applicability of tPA for acute ischemic stroke is insufficient to warrant its classification as standard of care.[101] Intra-arterial fibrinolysis, where a catheter is passed up an artery into the brain and the medication is injected at the site of thrombosis, has been found to improve outcomes in people with acute ischemic stroke.[102]
Mechanical thrombectomy

Another intervention for acute ischemic stroke is removal of the offending thrombus directly. This is accomplished by inserting a catheter into the femoral artery, directing it into the cerebral circulation, and deploying a corkscrew-like device to ensnare the clot, which is then withdrawn from the body. Mechanical embolectomy devices have been demonstrated effective at restoring blood flow in patients who were unable to receive thrombolytic drugs or for whom the drugs were ineffective,[103][104][105][106] though no differences have been found between newer and older versions of the devices.[107] The devices have only been tested on patients treated with mechanical clot embolectomy within eight hours of the onset of symptoms.
Angioplasty and stenting
Angioplasty and stenting have begun to be looked at as possible viable options in treatment of acute ischemic stroke. In a systematic review of six uncontrolled, single-center trials, involving a total of 300 patients, of intra-cranial stenting in symptomatic intracranial arterial stenosis, the rate of technical success (reduction to stenosis of <50%) ranged from 90-98%, and the rate of major peri-procedural complications ranged from 4-10%. The rates of restenosis and/or stroke following the treatment were also favorable.[108] This data suggests that a large, randomized controlled trial is needed to more completely evaluate the possible therapeutic advantage of this treatment.
Hemicraniectomy
Large territory strokes can cause significant edema of the brain with secondary brain injury in surrounding tissue. This phenomenon is mainly encountered in strokes of the middle cerebral artery territory, and is also called "malignant cerebral infaction" because it carries a dismal prognosis. Relief of the pressure may be attempted with medication, but some require hemicraniectomy, the temporary surgical removal of the skull on one side of the head. This confers a marked improvement in the risk of death, although some more people survive with disability who would otherwise have died.[109]
Secondary prevention of ischemic stroke
Anticoagulation can prevent recurrent stroke. Among patients with nonvalvular atrial fibrillation, anticoagulation can reduce stroke by 60% while antiplatelet agents can reduce stroke by 20%.[110] However, a recent meta-analysis suggests harm from anti-coagulation started early after an embolic stroke.[111] Stroke prevention treatment for atrial fibrillation is determined according to the CHADS/CHADS2 system. The most widely used anticoagulant to prevent thromboembolic stroke in patients with nonvalvular atrial fibrillation is the oral agent Warfarin while dabigatran is a new alternative which does not require prothrombin time monitoring.
If studies show carotid stenosis, and the patient has residual function in the affected side, carotid endarterectomy (surgical removal of the stenosis) may decrease the risk of recurrence if performed rapidly after stroke.
Hemorrhagic stroke
People with intracerebral hemorrhage require neurosurgical evaluation to detect and treat the cause of the bleeding, although many may not need surgery. Anticoagulants and antithrombotics, key in treating ischemic stroke, can make bleeding worse and cannot be used in intracerebral hemorrhage. Patients are monitored for changes in the level of consciousness, and their blood pressure, blood sugar, and oxygenation are kept at optimum levels.
Care and rehabilitation
Stroke rehabilitation is the process by which patients with disabling strokes undergo treatment to help them return to normal life as much as possible by regaining and relearning the skills of everyday living. It also aims to help the survivor understand and adapt to difficulties, prevent secondary complications and educate family members to play a supporting role.
A rehabilitation team is usually multidisciplinary as it involves staff with different skills working together to help the patient. These include nursing staff, physiotherapy, occupational therapy, speech and language therapy, and usually a physician trained in rehabilitation medicine. Some teams may also include psychologists, social workers, and pharmacists since at least one third of the patients manifest post stroke depression. Validated instruments such as the Barthel scale may be used to assess the likelihood of a stroke patient being able to manage at home with or without support subsequent to discharge from hospital.
Good nursing care is fundamental in maintaining skin care, feeding, hydration, positioning, and monitoring vital signs such as temperature, pulse, and blood pressure. Stroke rehabilitation begins almost immediately.
For most stroke patients, physical therapy (PT), occupational therapy (OT) and speech-language pathology (SLP) are the cornerstones of the rehabilitation process. Often, assistive technology such as a wheelchair, walkers, canes, and orthosis may be beneficial. PT and OT have overlapping areas of expertise, however PT focuses on joint range of motion and strength by performing exercises and re-learning functional tasks such as bed mobility, transferring, walking and other gross motor functions. Physiotherapists can also work with patients to improve awareness and use of the hemiplegic side. Rehabilitation involves working on the ability to produce strong movements or the ability to perform tasks using normal patterns. Emphasis is often concentrated on functional tasks and patient’s goals. One example physiotherapists employ to promote motor learning involves constraint-induced movement therapy. Through continuous practice the patient relearns to use and adapt the hemiplegic limb during functional activities to create lasting permanent changes.[112] OT is involved in training to help relearn everyday activities known as the Activities of daily living (ADLs) such as eating, drinking, dressing, bathing, cooking, reading and writing, and toileting. Speech and language therapy is appropriate for patients with the speech production disorders: dysarthria and apraxia of speech, aphasia, cognitive-communication impairments and/or dysphagia (problems with swallowing).
Patients may have particular problems, such as dysphagia, which can cause swallowed material to pass into the lungs and cause aspiration pneumonia. The condition may improve with time, but in the interim, a nasogastric tube may be inserted, enabling liquid food to be given directly into the stomach. If swallowing is still deemed unsafe, then a percutaneous endoscopic gastrostomy (PEG) tube is passed and this can remain indefinitely.
Treatment of spasticity related to stroke often involves early mobilisations, commonly performed by a physiotherapist, combined with elongation of spastic muscles and sustained stretching through various positioning.[16] Gaining initial improvements in range of motion is often achieved through rhythmic rotational patterns associated with the affected limb.[16] After full range has been achieved by the therapist, the limb should be positioned in the lengthened positions to prevent against further contractures, skin breakdown, and disuse of the limb with the use of splints or other tools to stabilize the joint.[16] Cold in the form of ice wraps or ice packs have been proven to briefly reduce spasticity by temporarily dampening neural firing rates.[16] Electrical stimulation to the antagonist muscles or vibrations has also been used with some success.[16]
Stroke rehabilitation should be started as quickly as possible and can last anywhere from a few days to over a year. Most return of function is seen in the first few months, and then improvement falls off with the "window" considered officially by U.S. state rehabilitation units and others to be closed after six months, with little chance of further improvement. However, patients have been known to continue to improve for years, regaining and strengthening abilities like writing, walking, running, and talking. Daily rehabilitation exercises should continue to be part of the stroke patient's routine. Complete recovery is unusual but not impossible and most patients will improve to some extent: proper diet and exercise are known to help the brain to recover.
Some current and future therapy methods include the use of virtual reality and video games for rehabilitation. These forms of rehabilitation offer potential for motivating patients to perform specific therapy tasks that many other forms do not.[113] Many clinics and hospitals are adopting the use of these off-the-shelf devices for exercise, social interaction and rehabilitation because they are affordable, accessible and can be used within the clinic and home.[113].
Other novel non-invasive rehabilitation methods are currently being developed to augment physical therapy to improve motor function of stroke patients, such as transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCS)[114] and robotic therapies.[115]
Prognosis
Disability affects 75% of stroke survivors enough to decrease their employability.[116] Stroke can affect patients physically, mentally, emotionally, or a combination of the three. The results of stroke vary widely depending on size and location of the lesion.[117] Dysfunctions correspond to areas in the brain that have been damaged.
Some of the physical disabilities that can result from stroke include muscle weakness, numbness, pressure sores, pneumonia, incontinence, apraxia (inability to perform learned movements), difficulties carrying out daily activities, appetite loss, speech loss, vision loss, and pain. If the stroke is severe enough, or in a certain location such as parts of the brainstem, coma or death can result.
Emotional problems resulting from stroke can result from direct damage to emotional centers in the brain or from frustration and difficulty adapting to new limitations. Post-stroke emotional difficulties include anxiety, panic attacks, flat affect (failure to express emotions), mania, apathy, and psychosis.
30 to 50% of stroke survivors suffer post stroke depression, which is characterized by lethargy, irritability, sleep disturbances, lowered self esteem, and withdrawal.[118] Depression can reduce motivation and worsen outcome, but can be treated with antidepressants.
Emotional lability, another consequence of stroke, causes the patient to switch quickly between emotional highs and lows and to express emotions inappropriately, for instance with an excess of laughing or crying with little or no provocation. While these expressions of emotion usually correspond to the patient's actual emotions, a more severe form of emotional lability causes patients to laugh and cry pathologically, without regard to context or emotion.[116] Some patients show the opposite of what they feel, for example crying when they are happy.[119] Emotional lability occurs in about 20% of stroke patients.
Cognitive deficits resulting from stroke include perceptual disorders, speech problems, dementia, and problems with attention and memory. A stroke sufferer may be unaware of his or her own disabilities, a condition called anosognosia. In a condition called hemispatial neglect, a patient is unable to attend to anything on the side of space opposite to the damaged hemisphere.
Up to 10% of all stroke patients develop seizures, most commonly in the week subsequent to the event; the severity of the stroke increases the likelihood of a seizure.[120][121]
Epidemiology
Stroke could soon be the most common cause of death worldwide.[122] Stroke is currently the second leading cause of death in the Western world, ranking after heart disease and before cancer,[2] and causes 10% of deaths worldwide.[123] Geographic disparities in stroke incidence have been observed, including the existence of a "stroke belt" in the southeastern United States, but causes of these disparities have not been explained.
The incidence of stroke increases exponentially from 30 years of age, and etiology varies by age.[124] Advanced age is one of the most significant stroke risk factors. 95% of strokes occur in people age 45 and older, and two-thirds of strokes occur in those over the age of 65.[118][23] A person's risk of dying if he or she does have a stroke also increases with age. However, stroke can occur at any age, including in childhood.
Family members may have a genetic tendency for stroke or share a lifestyle that contributes to stroke. Higher levels of Von Willebrand factor are more common amongst people who have had ischemic stroke for the first time.[125] The results of this study found that the only significant genetic factor was the person's blood type. Having had a stroke in the past greatly increases one's risk of future strokes.
Men are 25% more likely to suffer strokes than women,[23] yet 60% of deaths from stroke occur in women.[119] Since women live longer, they are older on average when they have their strokes and thus more often killed (NIMH 2002).[23] Some risk factors for stroke apply only to women. Primary among these are pregnancy, childbirth, menopause and the treatment thereof (HRT).
History

Episodes of stroke and familial stroke have been reported from the 2nd millennium BC onward in ancient Mesopotamia and Persia.[126] Hippocrates (460 to 370 BC) was first to describe the phenomenon of sudden paralysis that is often associated with ischemia. Apoplexy, from the Greek word meaning "struck down with violence," first appeared in Hippocratic writings to describe this phenomenon.[127][128]
The word stroke was used as a synonym for apoplectic seizure as early as 1599,[129] and is a fairly literal translation of the Greek term.
In 1658, in his Apoplexia, Johann Jacob Wepfer (1620–1695) identified the cause of hemorrhagic stroke when he suggested that people who had died of apoplexy had bleeding in their brains.[127][23] Wepfer also identified the main arteries supplying the brain, the vertebral and carotid arteries, and identified the cause of ischemic stroke [also known as cerebral infarction] when he suggested that apoplexy might be caused by a blockage to those vessels.[23]
Rudolf Virchow first described the mechanism of thromboembolism as a major factor.[130]
References
- ^ Sims NR, Muyderman H (2009). "Mitochondria, oxidative metabolism and cell death in stroke". Biochimica et Biophysica Acta. 1802 (1): 80–91. doi:10.1016/j.bbadis.2009.09.003. PMID 19751827.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k l m Donnan GA, Fisher M, Macleod M, Davis SM (2008). "Stroke". Lancet. 371 (9624): 1612–23. doi:10.1016/S0140-6736(08)60694-7. PMID 18468545.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Brain Basics: Preventing Stroke". National Institute of Neurological Disorders and Stroke. Retrieved 2009-10-24.
- ^ Shuaib A, Hachinski VC (1991). "Mechanisms and management of stroke in the elderly". CMAJ. 145 (5): 433–43. PMC 1335826. PMID 1878825.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Stam J (2005). "Thrombosis of the cerebral veins and sinuses". The New England Journal of Medicine. 352 (17): 1791–8. doi:10.1056/NEJMra042354. PMID 15858188.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Guercini F, Acciarresi M, Agnelli G, Paciaroni M (2008). "Cryptogenic stroke: time to determine aetiology". Journal of Thrombosis and Haemostasis. 6 (4): 549–54. doi:10.1111/j.1538-7836.2008.02903.x. PMID 18208534.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (1991). "Classification and natural history of clinically identifiable subtypes of cerebral infarction". Lancet. 337 (8756): 1521–6. doi:10.1016/0140-6736(91)93206-O. PMID 1675378.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) Later publications distinguish between "syndrome" and "infarct", based on evidence from imaging. "Syndrome" may be replaced by "hemorrhage" if imaging demonstrates a bleed. See Internet Stroke Center. "Oxford Stroke Scale". Retrieved 2008-11-14. - ^ Bamford JM (2000). "The role of the clinical examination in the subclassification of stroke". Cerebrovascular Diseases. 10 Suppl 4: 2–4. doi:10.1159/000047582. PMID 11070389.
- ^ Adams HP; Bendixen BH; Kappelle LJ; et al. (1993). "Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment". Stroke. 24 (1): 35–41. doi:10.1161/01.STR.24.1.35. PMID 7678184.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Goldstein LB, Simel DL (2005). "Is this patient having a stroke?". JAMA. 293 (19): 2391–402. doi:10.1001/jama.293.19.2391. PMID 15900010.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Harbison J, Massey A, Barnett L, Hodge D, Ford GA (1999). "Rapid ambulance protocol for acute stroke". Lancet. 353 (9168): 1935. doi:10.1016/S0140-6736(99)00966-6. PMID 10371574.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kidwell CS, Saver JL, Schubert GB, Eckstein M, Starkman S (1998). "Design and retrospective analysis of the Los Angeles Prehospital Stroke Screen (LAPSS)". Prehospital Emergency Care. 2 (4): 267–73. doi:10.1080/10903129808958878. PMID 9799012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kothari RU, Pancioli A, Liu T, Brott T, Broderick J (1999). "Cincinnati Prehospital Stroke Scale: reproducibility and validity". Annals of Emergency Medicine. 33 (4): 373–8. doi:10.1016/S0196-0644(99)70299-4. PMID 10092713.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b National Institute for Health and Clinical Excellence. Clinical guideline 68: Stroke. London, 2008.
- ^ Nor AM; Davis J; Sen B; et al. (2005). "The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument". Lancet Neurology. 4 (11): 727–34. doi:10.1016/S1474-4422(05)70201-5. PMID 16239179.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b c d e f O'Sullivan, Susan.B (2007). "Stroke". Physical Rehabilitation. Vol. 5. Philadelphia: F.A. Davis Company. p. 719.
{{cite book}}: Invalid|ref=harv(help); Unknown parameter|editors=ignored (|editor=suggested) (help) Cite error: The named reference "OSul07_719" was defined multiple times with different content (see the help page). - ^ "Thrombus".
- ^ "Circle of Willis".
- ^ "Brain anaurysm".
- ^ "Fibrinoid degeration".
- ^ Fisher, CM (1968-12-18). "The arterial lesions underlying lacunes". Acta Neuropathologica. 12 (1): 1–15. doi:10.1007/BF00685305. PMID 5708546.
- ^ "Sickle cell anemia".
- ^ a b c d e f g h i National Institute of Neurological Disorders and Stroke (NINDS) (1999). "Stroke: Hope Through Research". National Institutes of Health.
- ^ Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ (2005). "An evidence-based causative classification system for acute ischemic stroke". Annals of Neurology. 58 (5): 688–97. doi:10.1002/ana.20617. PMID 16240340.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Hypertension".
- ^ Miwa K; Hoshi T; Hougaku H; et al. (2010). "Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness". Intern. Med. 49 (9): 817–22. doi:10.2169/internalmedicine.49.3211. PMID 20453400.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ a b Herderscheê D, Hijdra A, Algra A, Koudstaal PJ, Kappelle LJ, van Gijn J (1992). "Silent stroke in patients with transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group". Stroke. 23 (9): 1220–4. doi:10.1161/01.STR.23.9.1220. PMID 1519274.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Leary MC, Saver JL (2003). "Annual incidence of first silent stroke in the United States: a preliminary estimate". Cerebrovasc. Dis. 16 (3): 280–5. doi:10.1159/000071128. PMID 12865617.
- ^ Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM (2002). "Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study". Stroke. 33 (1): 21–5. doi:10.1161/hs0102.101629. PMID 11779883.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Deb, P; Sharma, S; Hassan, KM (2010). "Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis". Pathophysiology : the official journal of the International Society for Pathophysiology / ISP. 17 (3): 197–218. doi:10.1016/j.pathophys.2009.12.001. PMID 20074922.
{{cite journal}}: Cite has empty unknown parameter:|author-name-separator=(help); Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Richard S. Snell (2006). Clinical neuroanatomy, 6. ed. Lippincott Williams & Wilkins, Philadelphia. pp. 478–485. ISBN 978-963-226-293-2.
- ^ Brunner and Suddarth's Textbook on Medical-Surgical Nursing, 11th Edition
- ^ Lees KR; Zivin JA; Ashwood T; et al. (2006). "NXY-059 for acute ischemic stroke". The New England Journal of Medicine. 354 (6): 588–600. doi:10.1056/NEJMoa052980. PMID 16467546.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b [1] Wang J.: Preclinical and clinical research on inflammation after intracerebral hemorrhage
- ^ Hill MD (2005). "Diagnostic biomarkers for stroke: a stroke neurologist's perspective". Clinical Chemistry. 51 (11): 2001–2. doi:10.1373/clinchem.2005.056382. PMID 16244286.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ World Health Organisation (1978). Cerebrovascular Disorders (Offset Publications). Geneva: World Health Organization. ISBN 92-4-170043-2. OCLC 4757533.
- ^ Kidwell CS, Warach S (2003). "Acute ischemic cerebrovascular syndrome: diagnostic criteria". Stroke. 34 (12): 2995–8. doi:10.1161/01.STR.0000098902.69855.A9. PMID 14605325.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chalela JA; Kidwell CS; Nentwich LM; et al. (2007). "Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison". Lancet. 369 (9558): 293–8. doi:10.1016/S0140-6736(07)60151-2. PMC 1859855. PMID 17258669.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Kidwell CS; Chalela JA; Saver JL; et al. (2004). "Comparison of MRI and CT for detection of acute intracerebral hemorrhage". JAMA. 292 (15): 1823–30. doi:10.1001/jama.292.15.1823. PMID 15494579.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b Straus SE, Majumdar SR, McAlister FA (2002). "New evidence for stroke prevention: scientific review". JAMA. 288 (11): 1388–95. doi:10.1001/jama.288.11.1388. PMID 12234233.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Goldstein LB; Adams R; Alberts MJ; et al. (2006). "Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline". Stroke. 37 (6): 1583–633. doi:10.1161/01.STR.0000223048.70103.F1. PMID 16675728.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b c NPS Prescribing Practice Review 44: Antiplatelets and anticoagulants in stroke prevention (2009). Available at nps.org.au
- ^ a b Antithrombotic Trialists' Collaboration (2002). "Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients". BMJ. 324 (7329): 71–86. doi:10.1136/bmj.324.7329.71. PMC 64503. PMID 11786451.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Medical Research Council Working Party (1985). "MRC trial of treatment of mild hypertension: principal results". British Medical Journal. 291 (6488): 97–104. doi:10.1136/bmj.291.6488.97. PMC 1416260. PMID 2861880.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thomson R (2009). "Evidence based implementation of complex interventions". BMJ. 339: b3124. doi:10.1136/bmj.b3124. PMID 19675081.
- ^ Hankey GJ (1999). "Smoking and risk of stroke". Journal of Cardiovascular Risk. 6 (4): 207–11. PMID 10501270.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wannamethee SG, Shaper AG, Whincup PH, Walker M (1995). "Smoking cessation and the risk of stroke in middle-aged men". JAMA. 274 (2): 155–60. doi:10.1001/jama.274.2.155. PMID 7596004.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Reynolds K; Lewis B; Nolen JD; et al. (2003). "Alcohol consumption and risk of stroke: a meta-analysis". JAMA. 289 (5): 579–88. doi:10.1001/jama.289.5.579. PMID 12578491.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Sloan MA, Kittner SJ, Rigamonti D, Price TR (1991). "Occurrence of stroke associated with use/abuse of drugs". Neurology. 41 (9): 1358–64. PMID 1891081.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.3945/ajcn.111.015115, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.3945/ajcn.111.015115instead. - ^ "Stroke Risk Factors". American Heart Association. 2007. Retrieved January 22, 2007.
- ^ Gorelick PB (1987). "Alcohol and stroke". Stroke; a Journal of Cerebral Circulation. 18 (1): 268–71. doi:10.1161/01.STR.18.1.268. PMID 3810763.
- ^ Westover AN, McBride S, Haley RW (2007). "Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients". Archives of General Psychiatry. 64 (4): 495–502. doi:10.1001/archpsyc.64.4.495. PMID 17404126.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cantu C, Arauz A, Murillo-Bonilla LM, López M, Barinagarrementeria F (2003). "Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs". Stroke. 34 (7): 1667–72. doi:10.1161/01.STR.0000075293.45936.FA. PMID 12791938.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ezekowitz JA, Straus SE, Majumdar SR, McAlister FA (2003). "Stroke: strategies for primary prevention". American Family Physician. 68 (12): 2379–86. PMID 14705756.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Ederle J, Brown MM (2006). "The evidence for medicine versus surgery for carotid stenosis". European Journal of Radiology. 60 (1): 3–7. doi:10.1016/j.ejrad.2006.05.021. PMID 16920313.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Whisnant JP (1996). "Effectiveness versus efficacy of treatment of hypertension for stroke prevention". Neurology. 46 (2): 301–7. PMID 8614485.
- ^ Collins R; Peto R; MacMahon S; et al. (1990). "Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context". Lancet. 335 (8693): 827–38. doi:10.1016/0140-6736(90)90944-Z. PMID 1969567.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Psaty BM; Lumley T; Furberg CD; et al. (2003). "Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis". JAMA. 289 (19): 2534–44. doi:10.1001/jama.289.19.2534. PMID 12759325.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ a b "Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration". Lancet. 346 (8991–8992): 1647–53. 1995. doi:10.1016/S0140-6736(95)92836-7. PMID 8551820.
- ^ Gueyffier F; Boissel JP; Boutitie F; et al. (1997). "Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators". Stroke. 28 (12): 2557–62. PMID 9412649.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Gueyffier F; Bulpitt C; Boissel JP; et al. (1999). "Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group". Lancet. 353 (9155): 793–6. doi:10.1016/S0140-6736(98)08127-6. PMID 10459960.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Staessen JA; Gasowski J; Wang JG; et al. (2000). "Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials". Lancet. 355 (9207): 865–72. doi:10.1016/S0140-6736(99)07330-4. PMID 10752701.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Beckett NS; Peters R; Fletcher AE; et al. (2008). "Treatment of Hypertension in Patients 80 Years of Age or Older". N. Engl. J. Med. 358 (18): 1887–98. doi:10.1056/NEJMoa0801369. PMID 18378519.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ a b Neal B, MacMahon S, Chapman N (2000). "Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration". Lancet. 356 (9246): 1955–64. doi:10.1016/S0140-6736(00)03307-9. PMID 11130523.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ The Allhat Officers And Coordinators For The Allhat Collaborative Research Group, (2002). "Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)". JAMA. 288 (23): 2981–97. doi:10.1001/jama.288.23.2981. PMID 12479763.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Wolf PA, Abbott RD, Kannel WB (1987). "Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study". Arch. Intern. Med. 147 (9): 1561–4. doi:10.1001/archinte.147.9.1561. PMID 3632164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fuster V; Rydén LE; Cannom DS; et al. (2006). "ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (7): e257–354. doi:10.1161/CIRCULATIONAHA.106.177292. PMID 16908781.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD (1989). "Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial". N. Engl. J. Med. 320 (14): 904–10. doi:10.1056/NEJM198904063201405. PMID 2619783.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b O'Regan C, Wu P, Arora P, Perri D, Mills EJ (2008). "Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients". Am. J. Med. 121 (1): 24–33. doi:10.1016/j.amjmed.2007.06.033. PMID 18187070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hebert PR, Gaziano JM, Hennekens CH (1995). "An overview of trials of cholesterol lowering and risk of stroke". Arch. Intern. Med. 155 (1): 50–5. doi:10.1001/archinte.155.1.50. PMID 7802520.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group". Lancet. 352 (9131): 837–53. 1998. doi:10.1016/S0140-6736(98)07019-6. PMID 9742976.
- ^ Dormandy JA; Charbonnel B; Eckland DJ; et al. (2005). "Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial". Lancet. 366 (9493): 1279–89. doi:10.1016/S0140-6736(05)67528-9. PMID 16214598.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Johnson ES, Lanes SF, Wentworth CE, Satterfield MH, Abebe BL, Dicker LW (1999). "A metaregression analysis of the dose-response effect of aspirin on stroke". Arch. Intern. Med. 159 (11): 1248–53. doi:10.1001/archinte.159.11.1248. PMID 10371234.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Hankey GJ, Sudlow CL, Dunbabin DW (2000). Hankey, Graeme (ed.). "Thienopyridine derivatives (ticlopidine, clopidogrel) versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients". Cochrane Database Syst Rev (2): CD001246. doi:10.1002/14651858.CD001246. PMID 10796426.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A (2006). "Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial". Lancet. 367 (9523): 1665–73. doi:10.1016/S0140-6736(06)68734-5. PMID 16714187.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Algra A; Halkes, PH; Van Gijn, J; Kappelle, LJ; Koudstaal, PJ; Algra, A (2007). "Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial". Lancet Neurol. 6 (2): 115–24. doi:10.1016/S1474-4422(06)70685-8. PMID 17239798.
- ^ Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA (2000). "Aspirin for the primary prevention of stroke and other major vascular events: meta-analysis and hypotheses". Arch. Neurol. 57 (3): 326–32. doi:10.1001/archneur.57.3.326. PMID 10714657.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bartolucci AA, Howard G (2006). "Meta-analysis of data from the six primary prevention trials of cardiovascular events using aspirin". Am. J. Cardiol. 98 (6): 746–50. doi:10.1016/j.amjcard.2006.04.012. PMID 16950176.
- ^ Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL (2006). "Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials". JAMA. 295 (3): 306–13. doi:10.1001/jama.295.3.306. PMID 16418466.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yerman T, Gan WQ, Sin DD (2007). "The influence of gender on the effects of aspirin in preventing myocardial infarction". BMC Med. 5: 29. doi:10.1186/1741-7015-5-29. PMC 2131749. PMID 17949479.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Rothwell PM; Eliasziw M; Gutnikov SA; et al. (2003). "Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis". Lancet. 361 (9352): 107–16. doi:10.1016/S0140-6736(03)12228-3. PMID 12531577.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Ringleb PA; Chatellier G; Hacke W; et al. (2008). "Safety of endovascular treatment of carotid artery stenosis compared with surgical treatment: a meta-analysis". J. Vasc. Surg. 47 (2): 350–5. doi:10.1016/j.jvs.2007.10.035. PMID 18241759.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Ederle J, Featherstone RL, Brown MM (2007). Brown, Martin M (ed.). "Percutaneous transluminal angioplasty and stenting for carotid artery stenosis". Cochrane Database Syst Rev (4): CD000515. doi:10.1002/14651858.CD000515.pub3. PMID 17943745.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ (2004). "Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery". Lancet. 363 (9413): 915–24. doi:10.1016/S0140-6736(04)15785-1. PMID 15043958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fairhead JF, Mehta Z, Rothwell PM (2005). "Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke". Neurology. 65 (3): 371–5. doi:10.1212/01.wnl.0000170368.82460.b4. PMID 16087900.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ U.S. Preventive Services Task Force (2007). "Screening for carotid artery stenosis: U.S. Preventive Services Task Force recommendation statement". Ann. Intern. Med. 147 (12): 854–9. PMID 18087056.
- ^ Halliday A; Mansfield A; Marro J; et al. (2004). "Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial". Lancet. 363 (9420): 1491–502. doi:10.1016/S0140-6736(04)16146-1. PMID 15135594.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Chambers BR, Donnan GA (2005). Chambers, Brian R (ed.). "Carotid endarterectomy for asymptomatic carotid stenosis". Cochrane Database Syst Rev (4): CD001923. doi:10.1002/14651858.CD001923.pub2. PMID 16235289.
- ^ Spence JD; Lees, K.; Spence, J. D. (2006). "Nutrition and stroke prevention". Stroke. 37 (9): 2430–5. doi:10.1161/01.STR.0000236633.40160.ee. PMID 16873712.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wang X; Qin X; Demirtas H; et al. (2007). "Efficacy of folic acid supplementation in stroke prevention: a meta-analysis". Lancet. 369 (9576): 1876–82. doi:10.1016/S0140-6736(07)60854-X. PMID 17544768.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Lonn E; Yusuf S; Arnold MJ; et al. (2006). "Homocysteine lowering with folic acid and B vitamins in vascular disease". N. Engl. J. Med. 354 (15): 1567–77. doi:10.1056/NEJMoa060900. PMID 16531613.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Toole JF; Malinow MR; Chambless LE; et al. (2004). "Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial". JAMA. 291 (5): 565–75. doi:10.1001/jama.291.5.565. PMID 14762035.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Heartscore.org
- ^ Saver JL (2006). "Time is brain - quantified". Stroke. 37 (1): 263–6. doi:10.1161/01.STR.0000196957.55928.ab. PMID 16339467.
- ^ Bellolio, MF (2011-09-07). Bellolio, M Fernanda (ed.). "Insulin for glycaemic control in acute ischaemic stroke". Cochrane database of systematic reviews (Online). 9 (9): CD005346. doi:10.1002/14651858.CD005346.pub3. PMID 21901697.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ The National Institute Of Neurological Disorders And Stroke Rt-Pa Stroke Study Group, (1995). "Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group". New England Journal of Medicine. 333 (24): 1581–7. doi:10.1056/NEJM199512143332401. PMID 7477192.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Wardlaw, JM (2009-10-07). Wardlaw, Joanna M (ed.). "Thrombolysis for acute ischaemic stroke". Cochrane database of systematic reviews (Online) (4): CD000213. doi:10.1002/14651858.CD000213.pub2. PMID 19821269.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ The European Cooperative Acute Stroke Study (ECASS), (2008). "Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke". New England Journal of Medicine. 359 (13): 1317–1329. doi:10.1056/NEJMoa0804656. PMID 18815396.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Dubinsky, R (2006). "Mortality of stroke patients treated with thrombolysis: analysis of nationwide inpatient sample". Neurology. 66 (11): 1742–1744. doi:10.1212/01.wnl.0000218306.35681.38. PMID 16769953.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Position Statement on the Use of Intravenous Thrombolytic Therapy in the Treatment of Stroke". American Academy of Emergency Medicine. Retrieved 2008-01-25.
- ^ Lee M, Hong KS, Saver JL (2010). "Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials". Stroke. 41 (5): 932–7. doi:10.1161/STROKEAHA.109.574335. PMID 20360549.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS (2007). "Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials". Stroke. 38 (4): 1274–80. doi:10.1161/01.STR.0000260187.33864.a7. PMID 17332445.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith WS; Sung G; Starkman S; et al. (2005). "Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial". Stroke. 36 (7): 1432–8. doi:10.1161/01.STR.0000171066.25248.1d. PMID 15961709.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Lutsep HL, Rymer MM, Nesbit GM (2008). "Vertebrobasilar revascularization rates and outcomes in the MERCI and multi-MERCI trials". J Stroke Cerebrovasc Dis. 17 (2): 55–7. doi:10.1016/j.jstrokecerebrovasdis.2007.11.003. PMID 18346645.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith WS (June 1, 2006). "Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I". AJNR Am J Neuroradiol. 27 (6): 1177–82. PMID 16775259.
- ^ Smith WS; Sung G; Saver J; et al. (2008). "Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial". Stroke. 39 (4): 1205–12. doi:10.1161/STROKEAHA.107.497115. PMID 18309168.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Derdeyn CP, Chimowitz MI (2007). "Angioplasty and Stenting for Atherosclerotic Intracranial Stenosis: Rationale for a Randomized Clinical Trial". Neuroimaging Clin. N. Am. 17 (3): 355–63, viii–ix. doi:10.1016/j.nic.2007.05.001. PMC 2040119. PMID 17826637.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Simard JM, Sahuquillo J, Sheth KN, Kahle KT, Walcott BP (2011). "Managing malignant cerebral infarction". Curr Treat Options Neurol. 13 (2): 217–29. doi:10.1007/s11940-010-0110-9. PMC 3243953. PMID 21190097.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hart RG, Pearce LA, Aguilar MI (2007). "Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation". Ann. Intern. Med. 146 (12): 857–67. PMID 17577005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paciaroni M, Agnelli G, Micheli S, Caso V (2007). "Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials". Stroke. 38 (2): 423–30. doi:10.1161/01.STR.0000254600.92975.1f. PMID 17204681.
{{cite journal}}: CS1 maint: multiple names: authors list (link) ACP JC synopsis - ^ O'Sullivan 2007, pp. 471, 484, 737, 740
- ^ a b B. Lange, S. Flynn & A. Rizzo, B.; Flynn, S.; Rizzo, A. (2009). "Initial usability assessment of off-the-shelf video game consoles for clinical game-based motor rehabilitation". Physical Therapy Reviews. 14 (5): 355–362. doi:10.1179/108331909X12488667117258.
- ^ Fregni, F (2007). "Technology Insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS". Nature Clinical Practice Neurology. 3 (7): 383–393. doi:10.1038/ncpneuro0530. PMID 17611487.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Balasubramanian, S (2010). "Robot-assisted rehabilitation of hand function". Curr Opin Neurol. 23 (6): 661–70. doi:10.1097/WCO.0b013e32833e99a4. PMID 20852421.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Coffey C. Edward, Cummings Jeffrey L, Starkstein Sergio, Robinson Robert (2000). Stroke - the American Psychiatric Press Textbook of Geriatric Neuropsychiatry (Second ed.). Washington DC: American Psychiatric Press. pp. 601–617.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stanford Hospital & Clinics. "Cardiovascular Diseases: Effects of Stroke". Retrieved 2005.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b Senelick Richard C., Rossi, Peter W., Dougherty, Karla (1994). Living with Stroke: A Guide for Families. Contemporary Books, Chicago. ISBN 0-8092-2607-3. OCLC 40856888 42835161.
{{cite book}}: Check|oclc=value (help)CS1 maint: multiple names: authors list (link) - ^ a b Villarosa, Linda, Ed., Singleton, LaFayette, MD, Johnson, Kirk A. (1993). Black Health Library Guide to Stroke. Henry Holt and Company, New York.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Reith J, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS (1997). "Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study". Stroke. 28 (8): 1585–9. doi:10.1161/01.STR.28.8.1585. PMID 9259753.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C (1997). "Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project". BMJ. 315 (7122): 1582–7. doi:10.1136/bmj.315.7122.1582. PMC 2127973. PMID 9437276.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Murray CJ, Lopez AD (1997). "Mortality by cause for eight regions of the world: Global Burden of Disease Study". Lancet. 349 (9061): 1269–76. doi:10.1016/S0140-6736(96)07493-4. PMID 9142060.
- ^ The World health report 2004. Annex Table 2: Deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002 (PDF). Geneva: World Health Organization. 2004.
- ^ Ellekjær, H (November 1, 1997). "Epidemiology of Stroke in Innherred, Norway, 1994 to 1996 : Incidence and 30-Day Case-Fatality Rate". Stroke. 28 (11): 2180–2184. doi:10.1161/01.STR.28.11.2180. PMID 9368561. Retrieved 2008-01-22.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bongers T; de Maat M; van Goor M; et al. (2006). "High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability". Stroke. 37 (11): 2672–7. doi:10.1161/01.STR.0000244767.39962.f7. PMID 16990571.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Ashrafian H (2010). "Familial stroke 2700 years ago". Stroke. 41 (4): e187. doi:10.1161/STROKEAHA.109.573170. PMID 20185778.
- ^ a b Thompson JE (August 1, 1996). "The evolution of surgery for the treatment and prevention of stroke. The Willis Lecture". Stroke. 27 (8): 1427–34. doi:10.1161/01.STR.27.8.1427. PMID 8711815.
- ^ Kopito, Jeff (2001). "A Stroke in Time". MERGINET.com. 6 (9).
{{cite journal}}: Unknown parameter|month=ignored (help) [dead link] - ^ R. Barnhart, ed. The Barnhart Concise Dictionary of Etymology (1995)
- ^ Schiller F (1970). "Concepts of stroke before and after Virchow". Med Hist. 14 (2): 115–31. PMC 1034034. PMID 4914683.
{{cite journal}}: Unknown parameter|month=ignored (help)
Further reading
- J. P. Mohr, Dennis Choi, James Grotta, Philip Wolf (2004). Stroke: Pathophysiology, Diagnosis, and Management. New York: Churchill Livingstone. ISBN 0-443-06600-0. OCLC 50477349 52990861.
{{cite book}}: Check|oclc=value (help)CS1 maint: multiple names: authors list (link) - Charles P. Warlow, Jan van Gijn, Martin S. Dennis, Joanna M. Wardlaw, John M. Bamford, Graeme J. Hankey, Peter A. G. Sandercock, Gabriel Rinkel, Peter Langhorne, Cathie Sudlow, Peter Rothwell (2008). Stroke: Practical Management (3rd ed.). Wiley-Blackwell. ISBN 1-4051-2766-X.
{{cite book}}: CS1 maint: multiple names: authors list (link)
