Acrylamide: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 66.87.71.94 to version by Whouk. False positive? Report it. Thanks, ClueBot NG. (1105953) (Bot) |
→Smoking cigarettes: Minor edit |
||
| Line 92: | Line 92: | ||
Acrylamide may be a natural decay product of the polyacrylamide used as a thickening agent in some commercial herbicides. Lab tests have shown that heat and light can decompose polyacrylamide into acrylamide.<ref>{{cite web|url=http://www.i-sis.org.uk/acrylamide.php|last=Cummins|first=Joe|title=Acrylamide In Cooked Foods: The Glyphosate Connection|publisher=Institute of Science in Society|date=2002-08-01|accessdate=2008-08-20}}</ref> |
Acrylamide may be a natural decay product of the polyacrylamide used as a thickening agent in some commercial herbicides. Lab tests have shown that heat and light can decompose polyacrylamide into acrylamide.<ref>{{cite web|url=http://www.i-sis.org.uk/acrylamide.php|last=Cummins|first=Joe|title=Acrylamide In Cooked Foods: The Glyphosate Connection|publisher=Institute of Science in Society|date=2002-08-01|accessdate=2008-08-20}}</ref> |
||
=== |
===Cigarettes=== |
||
[[tobacco smoking|Cigarette smoking]] is a major acrylamide source.<ref>[http://cebp.aacrjournals.org/cgi/content/abstract/16/11/2471 Assessment of the Relation between Biomarkers for Smoking and Biomarkers for Acrylamide Exposure in Humans]</ref> |
[[tobacco smoking|Cigarette smoking]] is a major acrylamide source.<ref>[http://cebp.aacrjournals.org/cgi/content/abstract/16/11/2471 Assessment of the Relation between Biomarkers for Smoking and Biomarkers for Acrylamide Exposure in Humans]</ref> |
||
Revision as of 19:11, 10 June 2012
| ||||

| ||||
| Names | ||||
|---|---|---|---|---|
| IUPAC name
prop-2-enamide
| ||||
| Identifiers | ||||
3D model (JSmol)
|
||||
| ChEBI | ||||
| ChEMBL | ||||
| ChemSpider | ||||
| ECHA InfoCard | 100.001.067 | |||
| KEGG | ||||
PubChem CID
|
||||
| UNII | ||||
CompTox Dashboard (EPA)
|
||||
| ||||
| ||||
| Properties | ||||
| C3H5NO | ||||
| Molar mass | 71.079 g·mol−1 | |||
| Density | 1.13 g/cm3 | |||
| Melting point | 84.5 °C (184.1 °F; 357.6 K) | |||
| Boiling point | - | |||
| 2.04 kg/L (25 °C) | ||||
| Hazards | ||||
| GHS labelling: | ||||
| class="wikitable collapsible" style="min-width: 50em;" | ||||
| Pictogram | Code | Symbol description | Image link | |
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- ‹The template Category link is being considered for merging.› Category:GHS templates
| Pictogram | Code | Symbol description | Image link | |
|---|---|---|---|---|
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- ‹The template Category link is being considered for merging.› Category:GHS templates[2]
|-
|-
| style="padding-left:1em;" |
| H301, H312, H315, H317, H319, H332, H340, H350, H361, H372[2]
|-
|-
| style="padding-left:1em;" |
| P201, P280, P301+P310, P305+P351+P338, P308+P313[2]
|- | NFPA 704 (fire diamond)
|
|- | Flash point | 138 °C
|-
| colspan=2 style="text-align:left; background:#f8eaba; border:1px solid #a2a9b1;" |
|-
|}
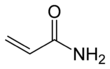
Acrylamide (or acrylic amide) is a chemical compound with the chemical formula C3H5NO. Its IUPAC name is prop-2-enamide. It is a white odourless crystalline solid, soluble in water, ethanol, ether, and chloroform. Acrylamide is incompatible[vague] with acids, bases, oxidizing agents, iron, and iron salts. It decomposes non-thermally to form ammonia, and thermal decomposition produces carbon monoxide, carbon dioxide, and oxides of nitrogen.
Acrylamide is prepared on an industrial scale by the hydrolysis of acrylonitrile by nitrile hydratase.
Most acrylamide is used to synthesize polyacrylamides, which find many uses as water-soluble thickeners. These include use in wastewater treatment, gel electrophoresis (SDS-PAGE), papermaking, ore processing, and the manufacture of permanent press fabrics. Some acrylamide is used in the manufacture of dyes and the manufacture of other monomers.
Acrylamide also occurs in many cooked starchy foods and is of concern as a possible carcinogen.[3] Acrylamide was accidentally discovered in foods in April 2002 by scientists in Sweden when they found the chemical in starchy foods, such as potato chips, French fries, and bread that had been heated (production of acrylamide in the heating process was shown to be temperature-dependent).[3] It was not found in food that had been boiled[3][4] or in foods that were not heated.[3]
In February 2009, Health Canada announced that they were assessing whether acrylamide, which occurs naturally during the cooking of French fries, potato chips, and other processed foods, is a hazard to human health and whether any regulatory action needs to be taken. They are currently collecting information on the properties and prevalence of acrylamide in order to make their assessment.[5] In December 2009, after a positive reception from the food industry, Health Canada invited comment from the public on this proposal.[6]
The European Chemical Agency added acrylamide to the list of substances of very high concern in March 2010.[7]
Laboratory use
Polyacrylamide was first used in a laboratory setting in the early 1950s. In 1959, the groups of Davis and Ornstein[8] and of Raymond and Weintraub[9] independently published on the use of polyacrylamide gel electrophoresis to separate charged molecules.[10] The technique is widely accepted today, and remains a common protocol in molecular biology labs.
Acrylamide has many other uses in molecular biology laboratories, including the use of linear polyacrylamide (LPA) as a carrier, which aids in the precipitation of small amounts of DNA. Many laboratory supply companies sell LPA for this use.[11]
Prepared foods
Acrylamide levels appear to rise as food is heated for longer periods of time. Though researchers are still unsure of the precise mechanisms by which acrylamide forms in foods, many believe it is a byproduct of the Maillard reaction. In fried or baked goods, acrylamide may be produced by the reaction between asparagine and reducing sugars (fructose, glucose, etc.) or reactive carbonyls at temperatures above 120 °C (248 °F).[12][13]
A study by the U.S. Food and Drug Administration (FDA) proposed a mechanism that involves asparagine, which, when heated in the presence of glucose, forms acrylamide.
Based on current stage of knowledge, acrylamide is a natural byproduct that forms when certain carbohydrate-rich foods are fried, baked, or roasted at temperatures above 120 °C. Acrylamide causes cancer in rats when administered orally in high-dose experiments, increasing tumors in the nervous system, oral cavity, peritoneum, thyroid gland, mammary gland, uterus, and clitoris.[14] There is a margin of 900-fold between the dose that gave cancer to 10% of rats and human exposure to acrylamide in the diet.[15]
Uncooked foods
Acrylamide has been found in black olives,[16] prunes,[17] and dried pears.[17]
Herbicides
Acrylamide may be a natural decay product of the polyacrylamide used as a thickening agent in some commercial herbicides. Lab tests have shown that heat and light can decompose polyacrylamide into acrylamide.[18]
Cigarettes
Cigarette smoking is a major acrylamide source.[19]
Beverages
Estimates for the proportion of acrylamide in adults’ diet coming from the consumption of coffee range from twenty to forty percent; prune juice has a high concentration of acrylamide, though adults consume it in far smaller quantities.[20]
Cooking methods that affect acrylamide production
Acrylamide cannot be created by boiling, and very few uncooked foods contain any detectable amounts.
Browning during baking, frying or deep-frying will produce acrylamide, and over-cooking foods may produce large amounts of acrylamide. The FDA has analyzed a variety of U.S. food products for levels of acrylamide since 2002. Results of the analysis can be found here.[21]
Reduction of acrylamide formation
The Confederation of the Food and Drink Industries in the EU (CIAA), (recently rebranded as FoodDrinkEurope) has published a number of brochures to help people reduce the amount of acrylamide formed in their food. They offer a general acrylamide "toolbox"[22] as well as publications specific to reducing the acrylamide in biscuits, crackers & crispbreads,[23] bread products,[24] breakfast cereals[25] potato crisps (chips),[26] and French fries.[27]
Storage
In the case of potatoes, for instance, the storage temperature should not drop below 8 °C (46 °F). When the temperature is as low as 4 °C (39 °F) the reducing sugar content rises sharply, so that the acrylamide formation during baking or deep-frying will be higher. [28]
Raw material
New varieties of potatoes that produce less or no acrylamide are being bred. Using varieties that are low in sugar such as sweet potatoes and blanching the raw potato before frying or roasting removes some of the sugars, thereby reducing the acrylamide in the end-product.[29] Experiments have also been conducted with oregano extract, which have resulted in a reduction of up to 49% of acrylamide in potatoes.[30]
Production methods
In many cases, it is advisable to lower the maximum temperature during baking. Also, new production methods such as vacuum frying may lower the acrylamide formation. When silicone is used as a foam inhibitor in deep-frying fats in the food industry, the acrylamide content is doubled.
Recipe formulation
Asparaginase, a naturally-occurring enzyme, can be added to bread or potato mixtures to reduce formation of acrylamide during cooking.[31]
Cations
Gökmen and Şenyuva (2007) showed that by dipping French fries into calcium chloride, they were able to reduce acrylamide formation by up to 95%. They reported that the treatment did not affect final product quality either. In similar manner, sodium ions were able to reduce acrylamide formation by as much as 50% in a model asparagine and fructose system.[32]
Some articles on the potential health risks to humans
Inhaled, absorbed or ingested acrylamide
There is evidence to suggest that exposure to large doses can cause damage to the male reproductive glands. Direct exposure to pure acrylamide by inhalation, skin absorption, or eye contact irritates the exposed mucous membranes, e.g., the nose, and can also cause sweating, urinary incontinence, nausea, myalgia, speech disorders, numbness, paresthesia, and weakened legs and hands. In addition, the acrylamide monomer is a potent neurotoxin, causing the disassembly or rearrangement of intermediate filaments.[33][34] Ingested acrylamide is metabolised to a chemically reactive epoxide, glycidamide.[35]
British Journal of Cancer
One study reanalysed a population-based Swedish case-control study encompassing cases with cancer of the large bowel, bladder, and kidney, and 538 healthy controls.[36] Researchers assessed the impact of dietary acrylamide “by linking extensive food frequency data with acrylamide levels in certain food items recorded by the Swedish National Food Administration. Unconditional logistic regression was used to estimate odds ratios, adjusting for potential confounders.” They “found consistently a lack of an excess risk, or any convincing trend, of cancer of the bowel, bladder, or kidney in high consumers of 14 different food items with a high (range 300–1200 µg/kg) or moderate (range 30–299 µg/kg) acrylamide content.” Also, “Unexpectedly, an inverse trend was found for large bowel cancer (P for trend 0.01) with a 40% reduced risk in the highest compared to lowest quartile.” The article concludes “We found reassuring evidence that dietary exposure to acrylamide in amounts typically ingested by Swedish adults in certain foods has no measurable impact on risk of three major types of cancer. It should be noted, however, that relation of risk to the acrylamide content of all foods could not be studied.”[36]
Cancer, epidemiology, biomarkers, and prevention
A number of studies pertaining to acrylamide have appeared in various issues of Cancer, Epidemiology, Biomarkers & Prevention. The first study included 62,573 women, aged 55–69 years.[37] The acrylamide intake of subcohort members and cases was assessed with a food frequency questionnaire and was based on chemical analysis of all relevant Dutch foods. Subgroup analyses were done for never-smokers to eliminate the influence of smoking, an important source of acrylamide. After 11.3 years of follow-up, the researchers observed 327, 300, and 1,835 cases of endometrial, ovarian, and breast cancer, respectively. They concluded that their subjects faced “increased risks of postmenopausal endometrial and ovarian cancer with increasing dietary acrylamide intake, in particular, among never-smokers. Risk of breast cancer was not associated with acrylamide intake.”[37]
The second study sought to evaluate how much of the acrylamide humans eat is absorbed by the body. The study consisted of six young healthy volunteers consuming a meal containing 0.94 mg of acrylamide, and then providing urine for up to 72 hours thereafter. The study concluded that “most of the acrylamide ingested with food is absorbed in humans.”[38]
Heat-generated food toxicants (HEATOX)
The Heat-generated Food Toxicants (HEATOX) Project was a “multidisciplinary research project involving 24 partners in 14 countries.” It ran from late 2003 to early 2007. Its objectives were to “estimate health risks that may be associated with hazardous compounds in heat-treated food [, and] find cooking/processing methods that minimise the amounts of these compounds, thereby providing safe, nutritious, and high-quality food-stuffs.”[39][40] It found that "the evidence of acrylamide posing a cancer risk for humans has been strengthened,"[41] and that "compared with many regulated food carcinogens, the exposure to acrylamide poses a higher estimated risk to European consumers."[39] HEATOX sought also to provide consumers with advice on how to lower their intake of acrylamide, specifically pointing out that home-cooked food tends to contribute far less to overall acrylamide levels than food that was industrially prepared, and that avoiding overcooking is one of the best ways to minimize exposure at home.[39] The report also recommended that national authorities highlight the following:
Potatoes low in sugar
- Low-sugar potato varieties
- Maintenance of suitable storage temperature during the supply chain
- Low sugar levels in prefabricated potato products for domestic frying.
Best frying temperature
- Frying temperature in the range 145 to 170 °C (293 to 338 °F) for deep-frying potatoes
- Clear and accurate cooking instruction on the package of pre-fried products
- Clear and accurate instruction for fryers for domestic use.
Golden, not brown!
- French fries and roasted potatoes cooked to a golden-yellow rather than golden-brown colour
- Bread toasted to the lightest colour acceptable.
International Journal of Cancer
In March, 2003, the International Journal of Cancer reported on a study conducted between 1991–2000 in Italy and Switzerland that analyzed the risk of cancer of the oral cavity and pharynx, esophagus, larynx, large bowel, breast, and ovaries.[42] It found “reassuring evidence for the lack of an important association between consumption of fried/baked potatoes and cancer risk.”[42]
More recently, in January, 2008, one of the HEATOX members published a study, stating “So far, epidemiological studies have not shown any association between human cancer risk and dietary exposure to acrylamide. The purpose of this study was to conduct a nested case control study within a prospective cohort study on the association between breast cancer and exposure to acrylamide using biomarkers.” The study found that “[a]fter adjustment for smoking behavior... a positive association was seen between acrylamide-hemoglobin levels and estrogen receptor positive breast cancer... A weak association between glycidamide hemoglobin levels and incidence of estrogen receptor positive breast cancer was also found, this association, however, entirely disappeared when acrylamide and glycidamide hemoglobin levels were mutually adjusted.”[43]
Journal of the American Medical Association
A 2005 study, published in JAMA, included 43,404 Swedish women in the Women’s Lifestyle and Health Cohort. The women’s greatest single source of acrylamide was from coffee (54% of intake), fried potatoes (12% of intake), and crisp bread (9% of intake). The study concluded that “Compared with the lowest quintile of acrylamide intake, there was no significantly increased risk of breast cancer in the higher quintiles and no evidence of a linear dose response. For quintile 5 compared with quintile 1, the relative risk was 1.19 (95% confidence interval, 0.91–1.55). Furthermore, there was no association between breast cancer risk and higher intake of any specific foods including coffee, fried potatoes, and crisp bread.”[44]
World Health Organization
The World Health Organization (WHO) has set up a clearinghouse for information about acrylamide that includes a database of researchers/data providers; references for research published elsewhere; information updates about the current status of research efforts; and updates on information relevant to the health risk of acrylamide in food.[45]
One question the site’s FAQ addresses is whether there can be an acceptable level of acrylamide in food. The WHO states that “Acrylamide belongs to the group of chemicals thought to have no reliably identifiable ‘threshold’ of effects, meaning that very low concentrations will also result in very low risks, but not in zero risk: Some risk is always present when the chemical is ingested. However, for these carcinogens, risk is thought to increase with increasing exposure. Very low risks (even of cancer), such as those that are less than one in one million, are considered to be acceptable to some consumers. To others this is unacceptable. The important pre-requisite for any decision is, however, a clear picture of the nature and level of the risk, as well as the potential for lowering this level. This clear picture does not exist for acrylamide at present.” [46]
American Journal of Epidemiology
The December 1, 2009, issue of the American Journal of Epidemiology included a study conducted at the Karolinska Institute in Stockholm concerning the relationship between dietary intake of acrylamide and breast cancer. Researchers found no statistically significant association between long-term dietary acrylamide intake and breast cancer. The study examined 61,433 Swedish women who were cancerfree and completed a food frequency questionnaire in 1987–1990 and again in 1997.[47]
On February 18, 2009, the same journal Advance Access published[48] a study by researchers at the Harvard School of Public Health and Brigham and Women’s Hospital in Boston, MA on the relationship between dietary acrylamide intake and premenopausal breast cancer. Similar to the Swedish study, the research revealed no association between dietary acrylamide intake and breast cancer risk. The study examined 90,628 premenopausal women.
European Journal of Cancer
The March 2009 issue of the European Journal of Cancer published a study examining the relationship between dietary intake of acrylamide and colorectal cancer. Conducted by Swedish researchers at the Karolinska Institute, this study of 45,306 men found no evidence of a link between dietary intake of acrylamide and risk for colorectal cancer.[49]
Safe levels of acrylamide in relation to neuropathy
In June 2002, the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) issued a report about the health implications of acrylamide in food. After study, the Consultation concluded that the "no observed adverse effect level" (NOAEL) for acrylamide neuropathy is 0.5 mg/kg body weight/day and the NOAEL for fertility changes is four times higher than for peripheral neuropathy. The study continued, “On the basis of current knowledge, controlling for peripheral neuropathy is expected to control for effects on fertility. The estimated average chronic human dietary intake is in the order of 1 μg/kg body weight/day. This provides a margin between exposure and the NOAEL of 500.”[50]
The WHO and FAO established that the safe limit of 0.5 mg/kg body weight/day pertains only to neuropathy. There has not been an established safe dietary limit of acrylamide as it pertains to causing cancer, since there is limited relative data.
Hence, a woman weighing 132 pounds (60 kg) could safely consume 30 mg of acrylamide daily without neuropathy; a man weighing 180 pounds (82 kg), about 41 mg; a child weighing 40 pounds (18 kg), 9 mg.[51]
Referring to the chart below for the amount of acrylamide in foods, in a single day, the child can eat 13 kg (29 lb) of French fried potatoes, the woman can drink 86 kg (~86 L, or 23 US gal) of prune juice, and the man can eat 29 kg (64 lb) of oven-baked potatoes, and each of them will have ingested less than 50 percent of the NOAEL of acrylamide.
| Food | AA concentration (μg/kg) |
Portion size (g) |
AA per portion (µg) |
|---|---|---|---|
| French fries (OB) | 698 | 70 | 48.8 |
| Prune juice | 174 | 140 | 24.4 |
| French fries (RF) | 334 | 70 | 23.3 |
| Postum | 93 | 240 | 22.3 |
| Potato chips | 546 | 30 | 16.4 |
| Canned black olives | 550 | 15 | 8.2 |
| Breakfast cereal | 131 | 55 | 7.3 |
| Brewed coffee | 8.5 | 240 | 3.2 |
(adapted from Table: Top Eight Foods by Acrylamide Per Portion, page 17)
Public awareness
On April 24, 2002, the Swedish National Food Administration announced that acrylamide can be found in baked and fried starchy foods, such as potato chips, breads, and cookies. Concern was raised mainly because of the probable carcinogenic effects of acrylamide. This was followed by a strong but short-lived interest from the press. On 2005-08-26, California attorney general Bill Lockyer filed a lawsuit against top makers of french fries and potato chips to warn consumers of the potential risk from consuming acrylamide.[52] The lawsuit was settled on 2008-08-01, with the food producers agreeing to cut acrylamide levels in half.[53]
In 2007, more than 100 articles were written about acrylamide, according to Nexis and Factiva, including pieces in the LA Times,[54] the Boston Globe,[55] the Guardian,[56] and the Wall Street Journal, among others. Of these articles, nearly half appeared in November and December, when people were frying potatoes for latkes, and roasting pigs and turkeys.
On August 1, 2008, four food manufacturers - H.J. Heinz Co., Frito-Lay, Kettle Foods Inc., and Lance Inc. - agreed to reduce levels of acrylamide in their products (such as potato chips and French fries) over a three-year period and pay a combined $3 million in fines as a settlement with the California attorney general's office. California had sued these four companies in 2005, alleging they violated a state requirement that companies post warning labels on products with carcinogens.[57]
See also
- Acrydite: research on this compound casts light on acrylamide
- Acrolein
- Deep-frying
- Deep fryer
- Vacuum fryer
- Substance of very high concern
References
- ^ a b "Globally Harmonized System of Classification and Labelling of Chemicals" (pdf). 2021. Annex 3: Codification of Statements and Pictograms (pp 268–385).
- ^ a b c Template:SigmaLink
- ^ a b c d Tareke E, Rydberg P.; et al. (2002). "Analysis of acrylamide, a carcinogen formed in heated foodstuffs". J. Agric. Food. Chem. 50 (17): 4998–5006. doi:10.1021/jf020302f. PMID 12166997.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|unused_data=ignored (help) - ^ Food Standards Agency, Acrylamide: your questions answered [1] Retrieved on 2008-01-01
- ^ Health Canada: Acrylamide
- ^ "Do you want anti-cancer drug in junk food?". The Star. Toronto. 2009-12-22. Retrieved 2010-04-23.
- ^ Candidate List of Substances of Very High Concern for authorisation
- ^ Davis and Ornstein
- ^ Raymond and Weintraub
- ^ Reynolds S, Weintraub L. (1959). "Acrylamide Gel as a Supporting Medium for Zone Electrophoresis". Science. 130 (3377): 711. doi:10.1126/science.130.3377.711. PMID 14436634.
- ^ Linear Polyacrylamide as a commercially sold DNA carrier
- ^ Mottram DS, Wedzicha BL. and Dodson AT. (2002). "Acrylamide is formed in the Maillard reaction". Nature. 419 (6906): 448–449. doi:10.1038/419448a. PMID 12368844.
{{cite journal}}: Unknown parameter|unused_data=ignored (help) - ^ Chemistry World, Acrylamide cancer link confirmed [2] Retrieved on 2008-01-01
- ^ Animal Test Results on Acrylamide in the Carcinogenic Potency Database
- ^ Comparing Possible Cancer Hazards from Human Exposures to Rodent Carcinogens
- ^ "Acrylamide detected in prune juice and olives" Food Safety & Quality Control Newsletter 26 March 2004, William Reed Business Media SAS, citing "Survey Data on Acrylamide in Food: Total Diet Study Results" United States Food and Drug Administration February 2004; later updated in June 2005, July 2006, and October 2006
- ^ a b Acrylamide in dried Fruits ETH Life (Swiss Federal Institute of Technology Zurich)
- ^ Cummins, Joe (2002-08-01). "Acrylamide In Cooked Foods: The Glyphosate Connection". Institute of Science in Society. Retrieved 2008-08-20.
- ^ Assessment of the Relation between Biomarkers for Smoking and Biomarkers for Acrylamide Exposure in Humans
- ^ page 17 Top Eight Foods by Acrylamide Per Portion
- ^ http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Acrylamide/ucm053549.htm
- ^ “toolbox”
- ^ biscuits, crackers & crispbreads
- ^ http://ec.europa.eu/food/food/chemicalsafety/contaminants/acrylamide/bread-EN-final.pdf
- ^ http://ec.europa.eu/food/food/chemicalsafety/contaminants/acrylamide/cereals-EN-final.pdf
- ^ potato crisps (chips)
- ^ http://ec.europa.eu/food/food/chemicalsafety/contaminants/acrylamide/frenchfries-EN-final.pdf
- ^ De Wilde, T; De Meulenaer, B; Mestdagh, F; Govaert, Y; Vandeburie, S; Ooghe, W; Fraselle, S; Demeulemeester, K; Van Peteghem, C; et al. (2005). "Influence of storage practices on acrylamide formation during potato frying". Journal of Agricultural and Food Chemistry. 53 (16): 6550–6557.
{{cite journal}}: Explicit use of et al. in:|first9=(help) - ^ Spivey, A. (2010). A matter of degrees: advancing our understanding of acrylamide. Environmental Health Perspectives, 118, 160(8). Retrieved October 20, 2010, from Health Reference Center-Academic database.
- ^ Ciesarová, Z., Kotsiou, K., Kukurová, K., Tasioula-Margari, M. (2010). Impact of oregano and virgin olive oil phenolic compounds on acrylamide content in a model system and fresh potatoes. Food Chemistry 123(4), 1149-55. Retrieved October 22, 2010, from ScienceDirect database.
- ^ Kornbrust, B. A. (September 17–20, 2006). "Enzymatic reduction of acrylamide formation using asparaginase from Aspergillus oryzae". World Grains Summit: Foods and Beverages. San Francisco, California USA.
{{cite conference}}: Unknown parameter|booktitle=ignored (|book-title=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gökmen, V., Şenyuva, H., (2007). Acrylamide formation is prevented by divalent cations during the Maillard Reaction. Food Chemistry 103, 196-203.
- ^ Kuperman AS. (1958). "Effects of acrylamide on the central nervous system of the cat". J. Pharmacol. Exp. Ther. 123 (3): 182–192. PMID 13564393.
- ^ Alberts, Lewis, Johnson, Raff, Roberts, and Walter,Molecular Biology of the Cell, 4th Edition, Routledge, March, 2002, ISBN 0-8153-3218-1
- ^ Joint FAO/WHO expert committee on food additives, Sixty-fourth meeting, Rome, 8–17 February 2005, Summary and conclusions Retrieved on 2008-01-01
- ^ a b Mucci, L A; Dickman, P W; Steineck, G; Adami, H-O; Augustsson, K (2003). "Dietary acrylamide and cancer of the large bowel, kidney, and bladder: Absence of an association in a population-based study in Sweden". British Journal of Cancer. 88 (1): 84. doi:10.1038/sj.bjc.6600726. PMC 2376776. PMID 12556964.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ a b Hogervorst, J. G.; Schouten, L. J.; Konings, E. J.; Goldbohm, R. A.; Van Den Brandt, P. A. (2007). "A Prospective Study of Dietary Acrylamide Intake and the Risk of Endometrial, Ovarian, and Breast Cancer". Cancer Epidemiology Biomarkers & Prevention. 16 (11): 2304. doi:10.1158/1055-9965.EPI-07-0581. PMID 18006919.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ Fuhr, U.; Boettcher, MI; Kinzig-Schippers, M; Weyer, A; Jetter, A; Lazar, A; Taubert, D; Tomalik-Scharte, D; Pournara, P (2006). "Toxicokinetics of Acrylamide in Humans after Ingestion of a Defined Dose in a Test Meal to Improve Risk Assessment for Acrylamide Carcinogenicity". Cancer Epidemiology Biomarkers & Prevention. 15 (2): 266. doi:10.1158/1055-9965.EPI-05-0647. PMID 16492914.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ a b c heat-Generated Food Toxicants; Identification, Characterisation and Risk Minimisation
- ^ HEATOX, Heat-generated food toxicants: identification, characterisation and risk minimisation
- ^ HEATOX project completed – brings new pieces to the Acrylamide Puzzle
- ^ a b Pelucchi, Claudio; Franceschi, Silvia; Levi, Fabio; Trichopoulos, Dimitrios; Bosetti, Cristina; Negri, Eva; La Vecchia, Carlo (2003). "Fried potatoes and human cancer". International Journal of Cancer. 105 (4): 558. doi:10.1002/ijc.11118. PMID 12712450.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ Olesen, PT; Olsen, A; Frandsen, H; Frederiksen, K; Overvad, K; Tjønneland, A (2008). "Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study". International Journal of Cancer. Journal International Du Cancer. 122 (9): 2094–100. doi:10.1002/ijc.23359. PMID 18183576.
- ^ Mucci, LA; Sandin, S; Bälter, K; Adami, HO; Magnusson, C; Weiderpass, E (2005). "Acrylamide intake and breast cancer risk in Swedish women". JAMA: the Journal of the American Medical Association. 293 (11): 1326–7. doi:10.1001/jama.293.11.1326. PMID 15769965.
- ^ Acrylamide, WHO
- ^ WHO|Frequently asked questions - acrylamide in food
- ^ Larsson, S. C.; Akesson, A.; Wolk, A. (2008). "Long-term Dietary Acrylamide Intake and Breast Cancer Risk in a Prospective Cohort of Swedish Women". American Journal of Epidemiology. 169 (3): 376. doi:10.1093/aje/kwn319. PMID 19015201.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ Wilson, K. M.; Mucci, L. A.; Cho, E.; Hunter, D. J.; Chen, W. Y.; Willett, W. C. (2009). "Dietary Acrylamide Intake and Risk of Premenopausal Breast Cancer". American Journal of Epidemiology. 169 (8): 954. doi:10.1093/aje/kwn421. PMC 2727226. PMID 19224978.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ Larsson, Susanna C.; Åkesson, Agneta; Bergkvist, Leif; Wolk, Alicja (2009). "Dietary acrylamide intake and risk of colorectal cancer in a prospective cohort of men". European Journal of Cancer. 45 (4): 513. doi:10.1016/j.ejca.2008.12.001. PMID 19121931.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ FAO/WHO Consultation on the Health Implications of Acrylamide in Food; Geneva, 25-27 June 2002, Summary Report
- ^ for these and all other unit conversions, see this)
- ^ Attorney General Lockyer Files Lawsuit to Require Consumer Warnings About Cancer-Causing Chemical in Potato Chips and French Fries, Office of the attorney general, State of California, Department of justice
- ^ Lawsuit over potato chip ingredients settled Retrieved on 2008-08-02
- ^ KFC to tell diners of chemical in potatoes - Los Angeles Times
- ^ Does a chemical formed in cooking french fries really cause cancer? - The Boston Globe
- ^ Atkins, Lucy (2007-12-04). "Is cooked food dangerous?". The Guardian. London. Retrieved 2010-04-23.
- ^ "Settlement will reduce carcinogens in potato chips". Associated Press. Archived from the original on 2008-08-21. Retrieved 2008-08-02.
External links
- Acrylamide Infonet
- Acrylamide Facts
- California Wants to Serve a Warning With Fries
- Center for Food Safety and Nutrition (cfsan.fda.gov) FAQ
- Court case about a suspected acrylamide poisoning
- Environmental Protection Agency Acrylamide and Food
- EPA releases a Toxicological Review of Acrylamide (External Review Draft) for public comment - 01/2008
- Girls who eat chips more likely to get breast cancer
- Harvard School of Public Health press release; baked and fried food does not increase risk of certain cancers in humans
- Harvard School of Public Health press release; no breast cancer risks from acrylamide via food
- IARC Monograph "Acrylamide."
- Information about Acrylamide from www.foodrisk.org
- International Chemical Safety Card 0091
- Institut national de recherche et de sécurité (1992). "Acrylamide." Fiche toxicologique n° 119. Paris:INRS. (in French)
- National Pollutant Inventory - Acrylamide
- NIOSH Pocket Guide to Chemical Hazards. "#0012". National Institute for Occupational Safety and Health (NIOSH).
- Prospective study of dietary acrylamide and risk of colorectal cancer among women
- Report about acrylamide in food and cancer risks
- World Health Organization