Triflusal: Difference between revisions
No edit summary |
No edit summary |
||
| Line 47: | Line 47: | ||
'''Triflusal''' is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a drug of the salicylate family but it is not a derivative of [[acetylsalicylic acid]] (ASA). Trade names include '''Disgren''', '''Grendis''', '''Aflen''' and '''Triflux''' <ref>Murdoch D, et al. Triflusal: a review of its use in cerebral infarction and myocardial infarction, and as thromboprophylaxis in atrial fibrillation.Drugs 2006; 66(5):671-92</ref> |
'''Triflusal''' is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a drug of the salicylate family but it is not a derivative of [[acetylsalicylic acid]] (ASA). Trade names include '''Disgren''', '''Grendis''', '''Aflen''' and '''Triflux''' <ref>Murdoch D, et al. Triflusal: a review of its use in cerebral infarction and myocardial infarction, and as thromboprophylaxis in atrial fibrillation.Drugs 2006; 66(5):671-92</ref> |
||
==Mechanism of action== |
==Mechanism of action== |
||
| Line 53: | Line 54: | ||
* preserves vascular prostacyclin, thus promoting anti-aggregant effect |
* preserves vascular prostacyclin, thus promoting anti-aggregant effect |
||
* blocks phosphodiesterase thereby increasing cAMP concentration, thereby promoting anti-aggregant effect due to inhibition of calcium mobilization |
* blocks phosphodiesterase thereby increasing cAMP concentration, thereby promoting anti-aggregant effect due to inhibition of calcium mobilization |
||
==Indication== |
==Indication== |
||
| Line 59: | Line 61: | ||
* Acute treatment of cerebral infarction, myocardial infarction |
* Acute treatment of cerebral infarction, myocardial infarction |
||
* Thromboprophylaxis due to atrial fibrillation |
* Thromboprophylaxis due to atrial fibrillation |
||
==Prevention of Stroke== |
==Prevention of Stroke== |
||
In the 2008 guidelines for stroke management from the European Stroke Organization, triflusal was for the first time recommended as lone therapy, as an alternative to acetylsalicylic acid plus dipyridamole, or clopidrogel alone for secondary prevention of atherothrombotic stroke. This recommendation was based on the doubleblind,randomised TACIP and TAPIRSS trials, which found triflusal to be equally as effective as aspirin in preventing post-stroke vascular events, while having a more favourable safety profile. |
In the 2008 guidelines for stroke management from the European Stroke Organization, triflusal was for the first time recommended as lone therapy, as an alternative to acetylsalicylic acid plus dipyridamole, or clopidrogel alone for secondary prevention of atherothrombotic stroke. This recommendation was based on the doubleblind,randomised TACIP and TAPIRSS trials, which found triflusal to be equally as effective as aspirin in preventing post-stroke vascular events, while having a more favourable safety profile.<ref>Matías-Guiu J, Ferro JM, Alvarez J, et al; for the TACIP Investigators. Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction. The TACIP study: a randomized, double-blind, multicenter trial. Stroke 2003; 34; 840-848</ref><ref>Culebras A, Rotta-Escalante R, Vila J, et al; and the TAPIRSS investigators. Triflusal vs. aspirin for prevention of cerebral infarction.A randomized stroke study. Neurology. 2004; 62:1073-1080</ref><ref>Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. The European Stroke Organization(ESO) Executive Committee and the ESO Writing Committee.Cerebrovasc Dis. 2008; 25: 57-507</ref> |
||
==Pharmacokinetics== |
|||
It is absorbed in the small intestine and its bio-availability ranges from 83% to 100%.<ref>Ramis J, Mis R, Conte L, Forn J. Rat and human plasma protein binding of the main metabolite of triflusal. Eur J Pharmacol.1990; 183: 1867-1868</ref><ref>Ramis J, Mis R, Forn J, Torrent J, Gorina E, Jané F. Pharmacokinetics of triflusal and its main metabolite HTB in healthy subjects following a single oral dose. Eur J Drug Metab Pharmacokinet.1991; 16: 169-273.37,38</ref> |
|||
==References== |
==References== |
||
Revision as of 14:27, 15 December 2012
This article needs more links to other articles to help integrate it into the encyclopedia. (December 2012) |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.726 |
| Chemical and physical data | |
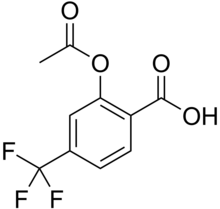
| Formula | C10H7F3O4 |
| Molar mass | 248.155 g/mol g·mol−1 |
| | |
Triflusal is a platelet aggregation inhibitor that was discovered and developed in the Uriach Laboratories, and commercialised in Spain since 1981. Currently, it is available in 25 countries in Europe, Asia, Africa and America. It is a drug of the salicylate family but it is not a derivative of acetylsalicylic acid (ASA). Trade names include Disgren, Grendis, Aflen and Triflux [1]
Mechanism of action
Triflusal is a selective platelet antiaggregant through;
- blocks cyclooxygenase inhibiting thromboxane A2, preventing aggregation
- preserves vascular prostacyclin, thus promoting anti-aggregant effect
- blocks phosphodiesterase thereby increasing cAMP concentration, thereby promoting anti-aggregant effect due to inhibition of calcium mobilization
Indication
Triflusal is indicated for;
- Prevention of cardiovascular events such as stroke
- Acute treatment of cerebral infarction, myocardial infarction
- Thromboprophylaxis due to atrial fibrillation
Prevention of Stroke
In the 2008 guidelines for stroke management from the European Stroke Organization, triflusal was for the first time recommended as lone therapy, as an alternative to acetylsalicylic acid plus dipyridamole, or clopidrogel alone for secondary prevention of atherothrombotic stroke. This recommendation was based on the doubleblind,randomised TACIP and TAPIRSS trials, which found triflusal to be equally as effective as aspirin in preventing post-stroke vascular events, while having a more favourable safety profile.[2][3][4]
Pharmacokinetics
It is absorbed in the small intestine and its bio-availability ranges from 83% to 100%.[5][6]
References
- ^ Murdoch D, et al. Triflusal: a review of its use in cerebral infarction and myocardial infarction, and as thromboprophylaxis in atrial fibrillation.Drugs 2006; 66(5):671-92
- ^ Matías-Guiu J, Ferro JM, Alvarez J, et al; for the TACIP Investigators. Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction. The TACIP study: a randomized, double-blind, multicenter trial. Stroke 2003; 34; 840-848
- ^ Culebras A, Rotta-Escalante R, Vila J, et al; and the TAPIRSS investigators. Triflusal vs. aspirin for prevention of cerebral infarction.A randomized stroke study. Neurology. 2004; 62:1073-1080
- ^ Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. The European Stroke Organization(ESO) Executive Committee and the ESO Writing Committee.Cerebrovasc Dis. 2008; 25: 57-507
- ^ Ramis J, Mis R, Conte L, Forn J. Rat and human plasma protein binding of the main metabolite of triflusal. Eur J Pharmacol.1990; 183: 1867-1868
- ^ Ramis J, Mis R, Forn J, Torrent J, Gorina E, Jané F. Pharmacokinetics of triflusal and its main metabolite HTB in healthy subjects following a single oral dose. Eur J Drug Metab Pharmacokinet.1991; 16: 169-273.37,38
