Cyclopropane: Difference between revisions
| Line 81: | Line 81: | ||
The triangular structure of cyclopropane requires the [[bond angle]]s between carbon-carbon bonds to be 60°. This is far less than the thermodynamically most stable angle of 109.5° (for bonds between atoms with sp<sup>3</sup> [[Orbital hybridisation|hybridised orbitals]]) and leads to significant [[ring strain]]. The molecule also has torsional strain due to the [[eclipsed conformation]] of its hydrogen atoms. As such, the bonds between the carbon atoms are considerably weaker than in a typical [[alkane]], resulting in much higher reactivity. |
The triangular structure of cyclopropane requires the [[bond angle]]s between carbon-carbon bonds to be 60°. This is far less than the thermodynamically most stable angle of 109.5° (for bonds between atoms with sp<sup>3</sup> [[Orbital hybridisation|hybridised orbitals]]) and leads to significant [[ring strain]]. The molecule also has torsional strain due to the [[eclipsed conformation]] of its hydrogen atoms. As such, the bonds between the carbon atoms are considerably weaker than in a typical [[alkane]], resulting in much higher reactivity. |
||
Bonding between the carbon centres is generally described by in terms of [[bent bond]]s.<ref>Eric V. Anslyn and Dennis A. Dougherty. ''Modern Physical Organic Chemistry.'' 2006. pages 850-852</ref> In this model the carbon-carbon bonds are bent outwards so that the '''inter-orbital angle''' is 104°. Doing this reduces the level of bond strain and is achieved by distorting the sp<sup>3</sup> hybridisation of carbon atoms to technically sp<sup>5</sup> hybridisation<ref>http://isites.harvard.edu/fs/docs/icb.topic93502.files/Lectures_and_Handouts/06-Handouts/deMeijere.pdf</ref>,<ref>http://isites.harvard.edu/fs/docs/icb.topic1032290.files/lecture%203.pdf</ref> (i.e. 1/6 s density and 5/6 p density) so that the C-C bonds have more p character than normal<ref>{{cite book|last=Knipe|first=edited by A.C.|title=March's advanced organic chemistry reactions, mechanisms, and structure.|year=2007|publisher=Wiley-Interscience|location=Hoboken, N.J.|isbn=0470084944|page=219|edition=6th ed.}}</ref> (at the same time the carbon-to-hydrogen bonds gain more s-character). One unusual consequence of bent bonding is that while the C-C bonds in are weaker than normal, the carbons atoms are also closer together than in a regular alkane bond: 151 [[picometer|pm]] versus 153 pm (average [[alkene]] bond: 146pm).<ref>{{cite journal|last=Allen|first=Frank H.|author2=Kennard, Olga |author3=Watson, David G. |author4=Brammer, Lee |author5=Orpen, A. Guy |author6= Taylor, Robin |title=Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds|journal=Journal of the Chemical Society, Perkin Transactions 2|year=1987|issue=12|pages=S1-S19|doi=10.1039/P298700000S1|url=http://pubs.rsc.org/en/content/articlelanding/1987/p2/p298700000s1}}</ref> |
Bonding between the carbon centres is generally described by in terms of [[bent bond]]s.<ref>Eric V. Anslyn and Dennis A. Dougherty. ''Modern Physical Organic Chemistry.'' 2006. pages 850-852</ref> In this model the carbon-carbon bonds are bent outwards so that the '''inter-orbital angle''' is 104°. Doing this reduces the level of bond strain and is achieved by distorting the sp<sup>3</sup> hybridisation of carbon atoms to technically sp<sup>5</sup> hybridisation<ref>http://isites.harvard.edu/fs/docs/icb.topic93502.files/Lectures_and_Handouts/06-Handouts/deMeijere.pdf</ref><sup>,</sup><ref>http://isites.harvard.edu/fs/docs/icb.topic1032290.files/lecture%203.pdf</ref> (i.e. 1/6 s density and 5/6 p density) so that the C-C bonds have more p character than normal<ref>{{cite book|last=Knipe|first=edited by A.C.|title=March's advanced organic chemistry reactions, mechanisms, and structure.|year=2007|publisher=Wiley-Interscience|location=Hoboken, N.J.|isbn=0470084944|page=219|edition=6th ed.}}</ref> (at the same time the carbon-to-hydrogen bonds gain more s-character). One unusual consequence of bent bonding is that while the C-C bonds in are weaker than normal, the carbons atoms are also closer together than in a regular alkane bond: 151 [[picometer|pm]] versus 153 pm (average [[alkene]] bond: 146pm).<ref>{{cite journal|last=Allen|first=Frank H.|author2=Kennard, Olga |author3=Watson, David G. |author4=Brammer, Lee |author5=Orpen, A. Guy |author6= Taylor, Robin |title=Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds|journal=Journal of the Chemical Society, Perkin Transactions 2|year=1987|issue=12|pages=S1-S19|doi=10.1039/P298700000S1|url=http://pubs.rsc.org/en/content/articlelanding/1987/p2/p298700000s1}}</ref> |
||
An alternative model for describing the bonding in cyclopropane involves [[Walsh diagram]]s and aims to do a better job fitting [[molecular orbital theory]] in light of spectroscopic evidence and group symmetry arguments. In this model cyclopropane is described as a [[three-center-bond]]ed{{disambiguation needed|date=November 2013}} orbital combination of methylene [[carbene]]s. |
An alternative model for describing the bonding in cyclopropane involves [[Walsh diagram]]s and aims to do a better job fitting [[molecular orbital theory]] in light of spectroscopic evidence and group symmetry arguments. In this model cyclopropane is described as a [[three-center-bond]]ed{{disambiguation needed|date=November 2013}} orbital combination of methylene [[carbene]]s. |
||
Revision as of 19:37, 9 August 2014
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cyclopropane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.771 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.08 g/mol | ||
| Density | 1.879 g/L (1 atm, 0 °C) | ||
| Melting point | −128 °C (−198 °F; 145 K) | ||
| Boiling point | −33 °C (−27 °F; 240 K) | ||
| Acidity (pKa) | ~46 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable Asphyxiant | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
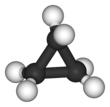
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms resulting in D3h molecular symmetry. Cyclopropane and propene have the same molecular formula but have different structures, making them structural isomers.
Cyclopropane is an anaesthetic when inhaled. In modern anaesthetic practice, it has been superseded by other agents, due to its extreme reactivity under normal conditions: When the gas is mixed with oxygen there is a significant risk of explosion.
History
Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the new substance in his first paper.[2] Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane.[3][4] The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium.[5] Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929;[6] industrial production had begun by 1936.[7]
Anaesthesia
Cyclopropane was introduced into clinical use by the American anaesthetist Ralph Waters who used a closed system with carbon dioxide absorption to conserve this then-costly agent. Cyclopropane is a relatively potent, non-irritating and sweet smelling agent with a minimum alveolar concentration of 17.5%[8] and a blood/gas partition coefficient of 0.55. This meant induction of anaesthesia by inhalation of cyclopropane and oxygen was rapid and not unpleasant. However at the conclusion of prolonged anaesthesia patients could suffer a sudden decrease in blood pressure, potentially leading to cardiac dysrhythmia; a reaction known as "cyclopropane shock".[9] For this reason, as well as its high cost and its explosive nature,[10] it was latterly used only for the induction of anaesthesia, before being largely phased out. Cylinders and flow meters were coloured orange.
Structure and bonding

The triangular structure of cyclopropane requires the bond angles between carbon-carbon bonds to be 60°. This is far less than the thermodynamically most stable angle of 109.5° (for bonds between atoms with sp3 hybridised orbitals) and leads to significant ring strain. The molecule also has torsional strain due to the eclipsed conformation of its hydrogen atoms. As such, the bonds between the carbon atoms are considerably weaker than in a typical alkane, resulting in much higher reactivity.
Bonding between the carbon centres is generally described by in terms of bent bonds.[11] In this model the carbon-carbon bonds are bent outwards so that the inter-orbital angle is 104°. Doing this reduces the level of bond strain and is achieved by distorting the sp3 hybridisation of carbon atoms to technically sp5 hybridisation[12],[13] (i.e. 1/6 s density and 5/6 p density) so that the C-C bonds have more p character than normal[14] (at the same time the carbon-to-hydrogen bonds gain more s-character). One unusual consequence of bent bonding is that while the C-C bonds in are weaker than normal, the carbons atoms are also closer together than in a regular alkane bond: 151 pm versus 153 pm (average alkene bond: 146pm).[15]
An alternative model for describing the bonding in cyclopropane involves Walsh diagrams and aims to do a better job fitting molecular orbital theory in light of spectroscopic evidence and group symmetry arguments. In this model cyclopropane is described as a three-center-bonded[disambiguation needed] orbital combination of methylene carbenes.
Synthesis
Cyclopropane was first produced via a Wurtz coupling, in which 1,3-dibromopropane was cyclised using sodium.[3] The yield of this reaction can be improved by exchanging the metal for zinc.[5]
- BrCH2CH2CH2Br + 2 Na → (CH2)3 + 2 NaBr
Cyclopropanation
Cyclopropane rings are found in numerous biomolecules and pharmaceutical drugs. As such the formation of cyclopropane rings, generally referred to as cyclopropanation, is an active area of chemical research.
Reactions
Owing to the increased π-character of its C-C bonds, cyclopropane can react like an alkene in certain cases. For instance it undergoes hydrohalogenation with mineral acids to give linear alkyl halides. Substituted cyclopropanes also react, following Markovnikov's rule.[16]
Safety
Cyclopropane is highly flammable. However, despite its strain energy it is not substantially more explosive than other alkanes.
See also
- Tetrahedrane contains four fused cyclopropane rings that form the faces of a tetrahedron
- Propellane contains three cyclopropane rings that share a single central carbon-carbon bond.
- Cyclopropene
- Methylenecyclopropane
References
- ^ Merck Index, 11th Edition, 2755.
- ^ Freund, August (1882) "Über Trimethylen" (On trimethylene), Monatshefte für Chemie … , 3 : 625-635.
- ^ a b August Freund (1881). "Über Trimethylen". Journal für Praktische Chemie. 26 (1): 625–635. doi:10.1002/prac.18820260125.
- ^ August Freund (1882). "Über Trimethylen". Monatshefte für Chemie. 3 (1): 625–635. doi:10.1007/BF01516828.
- ^ a b G. Gustavson (1887). "Ueber eine neue Darstellungsmethode des Trimethylens". J. Prakt. Chem. 36: 300–305. doi:10.1002/prac.18870360127. Cite error: The named reference "Gustavson" was defined multiple times with different content (see the help page).
- ^ G. H. W. Lucas and V. E. Henderson (1 August 1929). "A New Anesthetic: Cyclopropane : A Preliminary Report". Can Med Assoc J. 21 (2): 173–5. PMC 1710967. PMID 20317448.
- ^ H. B. Hass, E. T. McBee, and G. E. Hinds (1936). "Synthesis of Cyclopropane". Industrial & Engineering Chemistry. 28 (10): 1178–81. doi:10.1021/ie50322a013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eger, Edmond I.; Brandstater, Bernard; Saidman, Lawrence J.; Regan, Michael J.; Severinghaus, John W.; Munson, Edwin S. (1965). "Equipotent Alveolar Concentrations of Methoxyflurane, Halothane, Diethyl Ether, Fluroxene, Cyclopropane, Xenon and Nitrous Oxide in the Dog". Anesthesiology. 26 (6): 771–777. doi:10.1097/00000542-196511000-00012.
- ^ JOHNSTONE, M; Alberts, JR (July 1950). "Cyclopropane anesthesia and ventricular arrhythmias". British heart journal. 12 (3): 239–44. doi:10.1136/hrt.12.3.239. PMID 15426685.
- ^ MacDonald, AG (June 1994). "A short history of fires and explosions caused by anaesthetic agents". British journal of anaesthesia. 72 (6): 710–22. doi:10.1093/bja/72.6.710. PMID 8024925.
- ^ Eric V. Anslyn and Dennis A. Dougherty. Modern Physical Organic Chemistry. 2006. pages 850-852
- ^ http://isites.harvard.edu/fs/docs/icb.topic93502.files/Lectures_and_Handouts/06-Handouts/deMeijere.pdf
- ^ http://isites.harvard.edu/fs/docs/icb.topic1032290.files/lecture%203.pdf
- ^ Knipe, edited by A.C. (2007). March's advanced organic chemistry reactions, mechanisms, and structure (6th ed. ed.). Hoboken, N.J.: Wiley-Interscience. p. 219. ISBN 0470084944.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help) - ^ Allen, Frank H.; Kennard, Olga; Watson, David G.; Brammer, Lee; Orpen, A. Guy; Taylor, Robin (1987). "Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds". Journal of the Chemical Society, Perkin Transactions 2 (12): S1–S19. doi:10.1039/P298700000S1.
- ^ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. Jerry March ISBN 0-471-85472-7