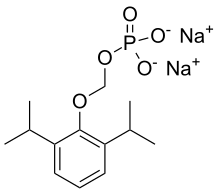
Fospropofol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Dependence liability | unknown |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 98%[1] |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | 0.81 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H21O5P |
| Molar mass | 288.280 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fospropofol (INN[3]), often used as the disodium salt (trade name Lusedra[4]) is an intravenous sedative-hypnotic agent. It is currently approved for use in sedation of adult patients undergoing diagnostic or therapeutic procedures such as endoscopy.
Clinical applications[edit]
Several water-soluble derivatives and prodrugs of the widely used intravenous anesthetic agent propofol have been developed, of which fospropofol has been found to be the most suitable for clinical development thus far.[5][6] Purported advantages of this water-soluble chemical compound include less pain at the site of intravenous administration, less potential for hyperlipidemia with long-term administration, and less chance for bacteremia.[citation needed] Often, fospropofol is administered in conjunction with an opioid such as fentanyl.[citation needed]
Clinical pharmacology[edit]
Mechanism of action[edit]
Fospropofol is a prodrug of propofol; as an organophosphate it is metabolized by alkaline phosphatases to phosphate and formaldehyde and the active metabolite, propofol.
Pharmacodynamics[edit]
Pharmacokinetics[edit]
Initial trial results on fospropofol pharmacokinetics were retracted by the investigators. As of 2011, new results were not available.[7]
Controlled substance[edit]
Fospropofol is classified as a Schedule IV controlled substance in the United States' Controlled Substances Act.[8]
See also[edit]
References[edit]
- ^ a b "LUSEDRA (fospropofol disodium) Injection" (PDF). Woodcliff Lake, New Jersey: Eisai Inc. October 2009. Archived from the original (PDF) on 22 November 2010. Retrieved 2 August 2010.
- ^ "Fospropofol disodium". PubChem Compound. Bethesda, Maryland: U.S. National Library of Medicine. Retrieved 9 February 2017.
- ^ "Recommended INNs 2006, pt 56" (PDF). World Health Organization. Retrieved 20 April 2016.
- ^ "FDA Approves Fospropofol and Follows ASAs Labeling Recommendation". American Society of Anesthesiologists. 2008-12-15. Archived from the original on 2011-05-26. Retrieved 2011-03-30.
- ^ Cooke A, Anderson A, Buchanan K, Byford A, Gemmell D, Hamilton N, et al. (April 2001). "Water-soluble propofol analogues with intravenous anaesthetic activity". Bioorganic & Medicinal Chemistry Letters. 11 (7): 927–930. doi:10.1016/S0960-894X(01)00088-9. PMID 11294393.
- ^ Bennett DJ, Anderson A, Buchanan K, Byford A, Cooke A, Gemmell DK, et al. (June 2003). "Novel water soluble 2,6-dimethoxyphenyl ester derivatives with intravenous anaesthetic activity". Bioorganic & Medicinal Chemistry Letters. 13 (12): 1971–1975. doi:10.1016/S0960-894X(03)00346-9. PMID 12781176.
- ^ Mahajan B, Kaushal S, Mahajan R (January 2012). "Fospropofol: pharmacokinetics?". Journal of Anaesthesiology Clinical Pharmacology. 28 (1): 134–135. doi:10.4103/0970-9185.92472. PMC 3275955. PMID 22345970.
- ^ "Schedule of Controlled Substances; Placement of Fospropofol into Schedule IV[permanent dead link]," 74 Federal Register 192 (October 6, 2009), pp. 51234–51236.
