Air well (condenser)


An air well or aerial well is a structure or device that collects water by promoting the condensation of moisture from air.[1] Designs for air wells are many and varied, but the simplest designs are completely passive, require no external energy source and have few, if any, moving parts.
An air well requires moisture in the air, but everywhere on Earth, even in the hottest climates, the atmosphere contains at least some water. According to a paper by Beysens and Milimouk: "The atmosphere contains 12,900 km3 (8,000 cubic miles) of fresh water, composed of 98% water vapour and 2% condensed water (clouds), a figure comparable to the renewable liquid water resources of inhabited lands (12,500 km3)."[2]
All air well designs provide a substrate with a temperature that is sufficiently low to allow dew to form. The process of condensation releases latent heat which must be dissipated in order for water collection to continue. The substrate must be cooled; this is typically achieved by radiating heat to the sky, by periods of cool breezes (particularly at night) or by conduction to some other heat sink.
There are three principal approaches to the design of air wells: high mass, radiative and active. Early in the twentieth century, there was interest in high-mass air wells, but despite much experimentation including the construction of massive structures, this approach proved to be a failure.[3] From the late twentieth century onward, there has been much investigation of low-mass, radiative collectors and these have proved to be much more successful. Also, since the invention of refrigeration systems there has been interest in active collectors that collect water in the same way as a dehumidifier; although these designs work well, they require an energy source which makes them uneconomical except in special circumstances. New, innovative designs seek to minimise the energy requirements of active condensers or make use of renewable energy resources.
A related, but quite distinct, technique of obtaining atmospheric moisture is the fog fence.
Air well is also an architectural term used to describe vertical shafts intended to provide passive ventilation in buildings in hot climates. In relation to this, also see windcatcher and solar chimney.
An air well should not be confused with a dew pond. A dew pond is an artificial pond intended for watering livestock. The name dew pond (sometimes cloud pond or mist pond) derives from the widely held belief that the pond was filled by moisture from the air.[4] In fact, dew ponds are primarily filled by rainwater.[5]
Dew
Dew is a form of precipitation that occurs naturally when atmospheric water vapour condenses onto a substrate. Dew is distinct from fog, in that fog is made of droplets of water that condense around particles in the air.
Air contains water vapour, most commonly reported as a relative humidity. How much water vapour air can contain depends on its temperature – warmer air can contain more water vapour than cooler air. When air is cooled to the dew point it becomes saturated and will condense on a suitable surface.[6] For instance, the dew temperature of air at 20° C (68° F) and 80% relative humidity is 18° C (64° F). The dew temperature falls to 10° C (50° F) if the relative humidity is only 25%.[2]
A stone mulch can significantly increase crop yields in arid areas. This is most notably the case in the Canary Islands: on the island of Lanzarote there is about 140 millimetres (5.5 in) of rain each year and there are no permanent rivers. Despite this, substantial crops can be grown by using a mulch of volcanic stones, a trick discovered after volcanic eruptions in 1730. Some credit the stone mulch with promoting dew; although the idea has inspired some thinkers, it seems unlikely that the effect is significant. Plants are able to absorb dew directly from their leaves, and the main benefit of a stone mulch is to reduce water loss from the soil and to eliminate competition from weeds.[7]
High mass collectors
A number of inventors experimented with high mass collectors. Of these, the most notable were Russian engineer Friedrich Zibold, French bioclimatologist Leon Chaptal, German-Australian researcher Wolf Klaphake and Belgian inventor Achille Knapen.
- Zibold’s collector
In 1900 near the site of the ancient Byzantine city of Theodosia, thirteen large piles of stones were discovered, each covering just over 900 square metres (9,700 sq ft) and about 10 metres (33 ft) tall. The finds were associated with the remains of 75 millimetres (3.0 in) diameter terracotta pipes which apparently led to wells and fountains in the city. Zibold concluded that the stacks of stone were condensers that supplied Theodosia with water. Zibold calculated that each air well produced more than 2,000 litres (440 imp gal; 530 US gal) each day (and more than 4500 litres under ideal conditions).[9]
To verify his hypothesis Zibold constructed a stone-pile condenser at an altitude of 288 metres (945 ft) on Mt. Tepe-Oba near the ancient site of Theodosia. Zibold’s condenser was surrounded by a wall 1 metre (3 ft 3 in) high, 20 metres (66 ft) wide, around a bowl-shaped collection area with drainage. He used sea stones 10–40 centimetres (3.9–15.7 in) in diameter piled 6 metres (20 ft) high in a truncated cone that was 8 metres (26 ft) in diameter across the top. The shape of the stone pile allowed a good air flow with only minimal thermal contact between the stones.[9]
Zibold's condenser began to operate in 1912 with a maximum daily production of 360 litres (79 imp gal; 95 US gal). The base developed leaks which forced the experiment to end in 1915 and the site was partially dismantled before being abandoned. (The site was rediscovered in 1993 and cleaned up.)[9] Zibold's condenser was of approximately the same size as the ancient stone piles that had been found and although the yield was significantly less than the yield Zibold had calculated for the original structures, the experiment was an inspiration for later developers.
- Chaptal’s collector
Inspired by Zibold's work, Chaptal built a small air well near Montpellier in 1929. Chaptal's condenser was a pyramidal concrete structure 3 metres (9.8 ft) square and 2.5 metres (8 ft 2 in) high, it was filled with 8 cubic metres (280 cu ft) of limestone pieces about 7.5 centimetres (3.0 in) in diameter. The pyramid had rings of small vent holes at the top and bottom which could be closed or opened as required to control the flow of air. The structure was allowed to cool during the night and then warm moist air was let in during the day. Dew formed on the limestone pieces and collected in a reservoir below ground level. The amount of water obtained varied from 1 litre (0.22 imp gal; 0.26 US gal) to 2.5 litres (0.55 imp gal; 0.66 US gal) per day depending on the atmospheric conditions.[9][10]
Chaptal did not consider his experiment a success. When he retired in 1946, he put the condenser out of order; possibly because he did not want to leave an improper installation to mislead those who might later continue studies on air wells.[11]
- Klaphake’s collectors
Klaphake was a successful chemist working in Berlin during the 1920s and 30s. During that time, he tested several forms of air wells in Yugoslavia and on Vis Island in the Adriatic Sea. Klaphake's work was inspired by the works of Maimonides, a known Jewish scholar who wrote in Arabic about 1,000 years ago and who mentioned the use of water condensers in Palestine.
Klaphake experimented with a very simple design: an area of mountain slope was cleared and smoothed with a watertight surface with a simple canopy supported by pillars or ridges. The sides of the structure were closed, but the top and bottom edges were left open. At nights, the mountain slope would cool and in the day moisture would collect on and run down the smoothed surface. Although the system apparently worked, it was expensive and Klaphake finally adopted a more compact design based on a masonry structure. This design was a sugarloaf-shaped building, about 15 metres (49 ft) high, with walls at least 2 metres (6 ft 7 in) thick, with holes on the top and at the bottom. The outer wall is made of concrete to give a high thermal capacity and the inner surface was made of a porous material such as sandstone. According to Klaphake:
The building produces water during the day and cools itself during the night; when the sun rises, the warm air is drawn through the upper holes into the building by the out-flowing cooler air, becomes cooled on the cold surface, deposits its water, which then oozes down and is collected somewhere underneath. It is wrong to think that this process works only on days with dew, as the inner surface becomes much cooler than one should expect. In Dalmatia, that day was a rare exception which failed to produce water.[12]
Traces of Klaphake's condensers have been tentatively identified.[13]
In 1935, Wolf Klaphake and his wife Maria emigrated to Australia. The Klaphake's decision to emigrate was probably primarily the result of Maria's encounters with the German secret police; their decision to settle in Australia (rather than, say, in Britain) was informed by Wolf's desire to develop a dew condenser. As a dry continent, Australia was likely to need alternative sources of fresh water and the Premier of South Australia, whom he had met in London, had expressed an interest. Klaphake made a specific proposal for a condenser at the small town of Cook where there was no supply of potable water. At Cook, the railway company had previously installed a large coal-powered active condenser, but it was prohibitively expensive to run, and it was cheaper to simply transport water. However, the Australian government turned down Klaphake's proposal and he lost interest in the project.[14]
- Knapen’s ariel well


Knapen,[15] who had previously worked on systems for removing damp from buildings,[16][17] was in turn inspired by Chaptal's work and he set about building an ambitiously large puits aerien (aerial well) on a 180 metres (590 ft) high hill at Trans-en-Provence in France. Beginning in 1930, Knapen's dew tower took 18 months to build; it still stands today, albeit in dilapidated condition. At the time of its construction, the condenser excited some public interest.[1]
The tower is 14 metres (46 ft) high and has massive masonry walls about 3 metres (9.8 ft) thick with a number of apertures to let in air. Inside there is a massive column made of concrete. At night, the whole structure is allowed to cool, and during the day warm moist air enters the structure via the high apertures, cools, descends, and leaves the building by the lower apertures.[18] Knapen’s intention was that water should condense on the cool inner column. In keeping with Chaptal’s finding that the condensing surface must be rough and the surface tension must be sufficiently low that the condensed water can drip, the central column's outer surface was studded with projecting plates of slate. The slates were placed nearly vertically to encourage dripping down to a collecting basin at the bottom of the structure.[9]
Unfortunately, the aerial well never achieved anything like its hoped-for performance and produced no more than a few litres of water each day.
Conclusions
None of the high-mass collectors performed well, and Knappen's ariel well was a particularly conspicuous failure.
Although ancient air wells are frequently mentioned in sources, there is scant evidence for them and persistent belief in their existence has the character of a modern myth.[11]
Zibold's collector apparently performed reasonably well, but in fact his exact results are not at all clear. Furthermore, it is now apparent that the mounds that Zibold identified as dew condensers were ancient burial mounds (a part of the necropolis of antic Theodosia) and that the pipes were medieval in origin and not associated with the mounds. If Zibold's condenser worked at all this was probably due to fact that a few stones near the surface of the mound were able to lose heat at night while being thermally isolated from the ground; however, it could never have produced the yield that Zibold envisaged.[11][19]
The essential problem with the high mass collectors was that they could not get rid of sufficient heat during the night - despite design features intended to ensure that this would happen.[9] While some thinkers have occasionally been persuaded that Zibold might have been on the right track after all,[1] the reasoning of an article in Journal of Arid Environments makes it clear that all high-mass condeser designs are doomed to failure:
We would like to stress the following point. To obtain condensation, the condenser temperature of the stones must be lower than the dew point temperature. When there is no fog, the dew point temperature is always lower than the air temperature. Meteorological data shows that the dew point temperature (an indicator of the water content of the air) does not change appreciably when the weather is stable. Thus wind, which ultimately imposes air temperature to the condenser, cannot cool the condenser to ensure its functioning. Another cooling phenomenon—radiative cooling—must operate. It is therefore at night-time, when the condenser cools by radiation, that liquid water can be extracted from air. It is very rare that the dew point temperature would increase significantly so as to exceed the stone temperature inside the stone heap. Occasionally, when this does happen, dew can be abundant during a short period of time. This is why subsequent attempts by L. Chaptal and A. Knapen to build massive dew condensers only rarely resulted in significant yields. [Emphasis as in original][11]
In retrospect, it may be seen as unfortunate that Zibold's pioneering work inspired so much fruitless effort. However, Zibold's legacy would inspire one more group of people, a party of academics who would go on to form the International Organization For Dew Utilization and, taking a quite different approach, they designed successful condensers.
Radiative collectors

By the end of the twentieth century, the details of how dew condenses was much better understood, the key insight being that low-mass collectors that rapidly lose heat by radiation perform best. A number of researchers worked on this method.[20]
In the 1960s, simple dew condensers made from sheets of polyethylene were used in Israel to irrigate plants, and in 1986 in New Mexico condensers made of a special foil produced sufficient water to supply young saplings.[2]
International organization for dew utilization

In 1992 a party of French academics attended a condensed matter conference in the Ukrain where physicist Daniel Beysens introduced them to the story of how ancient Theodosia was supplied with water from dew condensers. There were sufficiently intrigued that in 1993 they went to see for themselves. The supposed dew condensers turned out to be burial mounds, but they also found the remains of Zibold's condenser which they tidied up and examined closely. Fired with enthusiasm, the party returned to France and set up the International Organization For Dew Utilization (OPUR) with the specific objective of making dew available as an alternative source of water.[21][22]
OPUR began a study of dew condensation under laboratory conditions, they developed a special hydrophilic film and experimented with trial installations, including a 30 square metres (320 sq ft) collector in Corsica. Vital insights included the idea that the mass of the condensing surface should be as low as possible so that it could not easily retain heat; that is should be protected from unwanted thermal radiation by a layer of insulation; and that it should be hydrophilic so as to shed condensed moisture readily.
By the time they were ready for their first practical installation, they heard that one of their members, Girja Sharan had been given a grant to construct a dew condenser in Kothara, India. In April 2001, Girja Sharan had incidentally noticed substantial condensation on the roof of a cottage at Toran Beach Resort in the arid coastal region of Kutch, where he was briefly staying. The following year, he investigated the phenomenon more closely and interviewed local people. Financed by the Gujarat Energy Development Agency and the World Bank, Sharan and his team went on to developed passive, radiative condensers for use in the arid coastal region of Kutch.[23] Active commercialisation began in 2006.[24]
Sharan tested a wide range of materials and got good results from galvanised iron and aluminium sheets, but found that sheets of a special plastic developed by the OPUR just 400 micrometres (0.016 in) thick generally worked even better than the metal sheets and were less expensive.[25] The plastic film, known as OPUR foil, is hydrophilic and is made from polyethylene mixed with titanium oxide and barium sulphate.

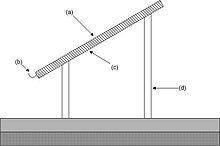
A typical radiative collector presents a condensing surface at an angle of 30° from the horizontal. The condensing surface is backed by a thick layer of insulating material such as polystyrene foam and supported 2–3 metres (7–10 ft) above ground level. Such condensers may be conveniently installed on the ridge roofs of low buildings or supported by a simple frame. Although other heights do not typically work quite so well, it may be less expensive or more convenient to mount a collector near to ground level on a two-storey building.
A radiative collector should be thermally isolated from any mass, including the ground. The condensing surface should be left open to radiate heat into space and should be well away from any source of heat or anything that can reflect heat radiation into it. Ideally, the condensing surface should be well wetted to reduce the nucleation barrier.[26]
The 600 square metres (6,500 sq ft) radiative condenser illustrated near the start of this article is built near the ground. In the area of north-west India where it is installed dew occurs for 8 months a year, and the installation collects about 15 millimetres (0.59 in) of dew water over the season with nearly 100 dew-nights. In a year it provides a total of about 9 cubic metres (320 cu ft) of potable water for the school which owns and operates the site.
Although flat designs have the benefit of simplicity, other designs such as inverted pyramids and cones can be significantly more effective. This is probably because the designs shield the condensing surfaces from unwanted heat radiated by the lower atmosphere and being symmetrical they are not sensitive to wind direction.[27]
New materials may make even better collectors.[28] One such material is inspired by the Namib Desert beetle, which survives only on the moisture it extracts from the atmosphere. It has been found that its back is coated with microscopic projections: the peaks are hydrophilic and the troughs are hydrophobic.[29][30] Researchers at the Massachusetts Institute of Technology have emulated this capability by creating a textured surface that combines alternating hydrophobic and hydrophilic materials.
Active collectors
This article or section is in a state of significant expansion or restructuring. You are welcome to assist in its construction by editing it as well. If this article or section has not been edited in several days, please remove this template. If you are the editor who added this template and you are actively editing, please be sure to replace this template with {{in use}} during the active editing session. Click on the link for template parameters to use.
This article was last edited by Everchanging02 (talk | contribs) 15 years ago. (Update timer) |
In the 1930s, American designers added condenser systems to airships to collect moisture from engine exhausts. The collected moisture was used as ballast and was collected in order to compensate for the loss of weight as fuel was consumed. By collecting ballast in this way, the airship's buoyancy could be kept constant without having to release helium gas which was both expensive and in finite supply.[31]
There are a number of modern, innovative designs that minimise the energy requirements of condensers. One method is to use cold seawater pumped up a depth of about 500 metres (1,600 ft) where the water temperature may be as low as about 4 °C (39 °F). This is essentially the method used in the Seawater Greenhouse that uses cool seawater to both cool and humidify the interior of greenhouse-like structure. The cooling can be so effective that not only do the plants inside benefit from an artificial dew-fall, but dew collects on the outside of the structure and can easily be collected by gutters.[2]
Another type of atmospheric water collector makes use of desiccants which adsorb atmospheric water at ambient temperature. Systems of this sort have proved to be very useful as emergency supplies of safe drinking water.[32][33] For regeneration, the desiccant needs to be heated, but in some designs this energy is supplied by the sun: air is ventilated at night over a bed of desiccants that adsorb the water vapour. During the day, the premises are closed, the greenhouse effect increases the temperature and, as in solar desalination pools, the water vapour is partially desorbed, condenses on a cold part and is collected.[2]
References
- ^ a b c "Air Well Waters Parched Farms". Popular Science. 122 (3). Bonnier Corporation. 1933. ISSN 0161-7370.
{{cite journal}}: Cite has empty unknown parameters:|laydate=,|laysummary=,|day=,|laysource=, and|coauthors=(help); Unknown parameter|month=ignored (help) Cite error: The named reference "popular science 1933" was defined multiple times with different content (see the help page). - ^ a b c d e D. Beysens and I. Milimouk (December). "The Case For Alternative Fresh Water Sources" (PDF). International Organization For Dew Utilization. Retrieved 2009-03-10.
{{cite web}}: Check date values in:|date=and|year=/|date=mismatch (help); Cite has empty unknown parameters:|dateformat=and|month=(help) - ^ Alton, 2003. p. 1014.
- ^ Oxford English Dictionary: "dew-pond"
- ^ Pugsley, 1939
- ^ Naval Meteorology and Oceanography Command (2007). Atmospheric Moisture. United States Navy. Retrieved on 2008-12-27.
- ^
Pearce, Fred (2006). "The Miracle of the Stones". New Scientist: 50–51.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help); Unknown parameter|quotes=ignored (help) - ^ Based on diagram by Nikolayev et all, 1996
- ^ a b c d e f Nelson, 2003
- ^ Hills, 1966. p. 232.
- ^ a b c d Beysens et al 2006
- ^ Klaphake quoted by Nelson, 2003
- ^
"In Croatia". OPUR. Retrieved 17 March 2009.
{{cite web}}: Cite has empty unknown parameter:|dateformat=(help) - ^
"Wolf Klaphake". Uncommon Lives (National Archives of Australia). Retrieved 2009-03-17.
{{cite web}}: Cite has empty unknown parameters:|dateformat=and|month=(help) - ^
"British Knapen - The Early Years" (pdf). ProTen Services. Retrieved 11 March 2009.
{{cite web}}: Cite has empty unknown parameter:|dateformat=(help) - ^ Prevention Of Damp In Buildings. The Manchester Guardian, 27 February 1930 p. 6 column F.
- ^
"ProTen Services Celebrates 80 Years of Service" (pdf). ProTen Services. Retrieved 11 March 2009.
{{cite web}}: Cite has empty unknown parameter:|dateformat=(help) - ^
Achile Knappen. "Improved means for collecting moisture from the atmosphere". European Patent Office. Retrieved 2009-04-28.
{{cite web}}: Cite has empty unknown parameter:|dateformat=(help) - ^ Nikolayev et al, 1996
- ^ Sharan, 2006. p. 22.
- ^ OPUR Ou la Conquete de la Rosee - The Conquest of Dew (in French with English subtitles). on YouTube
- ^
"International Organization For Dew Utilization". Retrieved 27 April 2009.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Sharan, 2006. Acknowledgement
- ^ Mukund, Dixit (1 April). "Leveraged Innovation Management: Key Themes from the Journey of Dewrain Harvest Systems" (pdf). Retrieved 30 March 2009.
{{cite web}}: Check date values in:|date=and|year=/|date=mismatch (help); Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sharan, 2006. p. 27.
- ^
Sharan, Girja. "Dew Yield From Passive Condensers in a Coastal Arid Area - Kutch" (pdf). p. 2. Retrieved 30 March 2009.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help); More than one of|author=and|last=specified (help) - ^ Clus, et al.
- ^ Sharan, 2006. p. 20.
- ^
Parker, A. R. & C. R. Lawrence (2001). "Water capture by a desert beetle". Nature. 414: 33–34. doi:10.1038/35102108.
{{cite journal}}: Unknown parameter|quotes=ignored (help) - ^ Harries-Rees, Karen (August 31, 2005). "Desert beetle provides model for fog-free nanocoating". Chemistry World News. Royal Society of Chemistry.
- ^ Allen, 1931. p. 37.
- ^
"Aqua Sciences". Retrieved 29 April.
{{cite web}}: Check date values in:|accessdate=(help); Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Pure water ... from thin Air! on YouTube – KBTV: Aqua Sciences Emergency Water Station, that produces pure water out of the humidity-saturated air in the wake of a hurricane.
Further reading
- Allen, Hugh (1931). The Story of the Airship. Goodyear Tire and Rubber Company.
{{cite book}}: Cite has empty unknown parameter:|coauthor=(help) - Alton Stewart, Bobby (2003). Encyclopedia of water science. Marcel Dekker. ISBN 9780824709488.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - Beysens, D. (2006). "Comment on "The moisture from the air as water resource in arid region: Hopes, doubt and facts" by Kogan and Trahtman" (PDF). Journal of Arid Environments. Elsevier. doi:10.1016/j.jaridenv.2006.01.011.
{{cite journal}}: Cite has empty unknown parameters:|laydate=,|laysummary=,|day=,|month=, and|laysource=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Clus, Owen. "Radiation-cooled Dew Water Condensers Studied by Computational Fluid Dynamic (CFD)" (pdf). Retrieved 30 March 2009.
{{cite web}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Hills, Edwin Sherbon (1966). Arid Lands: A Geographical Appraisal. Methuen.
{{cite book}}: Cite has empty unknown parameter:|coauthor=(help) - Nelson, Robert A. (2003). "Air Wells, Fog Fences & Dew Ponds – Methods for Recovery of Atmospheric Humidity". Rex Research. Retrieved 2009-03-10.
This article has been widely reproduced, including extracts in Sharan, 2006.
{{cite web}}: Cite has empty unknown parameters:|dateformat=and|month=(help) - Nikolayev, V.S. (1996). "Water Recovery from Dew". Journal of Hydrology (182). Elsevier.
{{cite journal}}: Cite has empty unknown parameters:|laydate=,|laysummary=,|day=,|month=, and|laysource=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Pugsley, Alfred J (1939). Dewponds in Fable and Fact. London: Country Life Ltd.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Sharan, Girja (2006). Dew Harvest. Foundation Books. ISBN 81-7596-326-3.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Sharan, Girja (2007). "Harvesting dew to supplement drinking water supply in arid coastal villages of Gujarat" (pdf). Indian Institute of Management. Retrieved 2009-03-17.
{{cite web}}: Cite has empty unknown parameters:|dateformat=and|month=(help) - Lindsley, E.F. (1984). "Airwell extracts Pure Water From the Air". Popular Science. 224 (1). Bonnier Corporation.
{{cite journal}}: Cite has empty unknown parameters:|laydate=,|laysummary=,|day=,|laysource=, and|coauthors=(help); Unknown parameter|month=ignored (help)
External links
- OPUR in India (visually self-explanatory, in English and other languages with French subtitles). on YouTube
- "Fog Quest Official Site". Retrieved 2009-03-19.
{{cite web}}: Cite has empty unknown parameters:|dateformat=and|month=(help) - "Dew Harvesting". Rainwater Harvesting Guide. Retrieved 2009-03-19.
{{cite web}}: Cite has empty unknown parameters:|dateformat=and|month=(help)
