Talk:Hemoglobin
| Medicine B‑class High‑importance | ||||||||||
| ||||||||||
Suggest that a pointer to Proteopedia's Hemoglobin page be added to the structure section, see, specifically: http://www.proteopedia.org/wiki/index.php/Hemoglobin Looking forward to hearing your reaction to this 3D graphic view integrating the text with 3D structure of hemoglobin.
Paragraph four
Paragraph four wants attention: it's a pointless distinction between "broken down" and "broken up." But the paragraph makes it sound as if the hemoglobin and a red blood cell are always recycled at the same time. So it would seem that each red blood cell has a certain amount of hemoglobin that stays with it for the duration of that cell's life? Is that the case? (interesting, but not as cute as monogamous ducks.) :-D
Point taken. I hope it's a bit clearer now. On your other point, human red blood cells I believe are pretty much stuck with their initial load of hemoglobin. Mammalian red blood cells don't have cell nuclei, so they can't be doing much de novo synthesis of any protein. Reptile blood cells do have nuclei (or Jurassic Park would have lost its major plot premise), so they might do synthesis. I'm not a herpetologist so I don't know.
- Just a side note: The classification of Dinosaurs is a hotly debated topic. They are not what we would call reptiles because of their activity levels and other physio/morpho-logical differences that can be inferred/ascertained from the fossil record. In fact they are closer to birds in many respects. So we can not say whether or not the RBCs of of dinos had nuclei or not. However, all blood from almost every animal has white blood cells in it, that almost without expection all have nuclei with at least some DNA. maveric149
The paragraph recently moved to the bottom probably should be used as the introduction to a new topic called oxygen transport. - Dwmyers
I just added the part about carbon dioxide. I know that it is not constructed very well but I thought it was an important point. Do you think the bohr effect deserves a page of it own? or should it just be defined in this article? DiJay
Question: Since heme is formed in the mitochondria, and mammalian erythrocytes do not have mitochondria in order to provide more space for hemoglobin, how exactly is hemoglobin made for the red blood cell? Especially without a nucleus to create any mRNA.
- Immature mammalian erythrocytes have both nuclei and mitochondria: the hemoglobin carried in the mature cell is made in the immature cell, before it loses them. - Nunh-huh 04:07, 22 Oct 2004 (UTC)
Article structure and HB chemical representation
is it better to have structure and synthesis together? The edit about chemical formula of Hb is not accurate. Does it refer to HbA, HbA2, HbF which have varying numbers of amino acids? " Hemoglobin is chemically represented by (C2952H4664N812O832S8Fe4). "
As I see it, the empirical formula C738H1166N812O203S2Fe is not correct. This because it doesn't add up if you multiply it by four (which I assume the original formula was divided with).Yfé (talk) 09:37, 1 December 2008 (UTC)
Decay Time
How long does it take for hemoglobin to decay if blood falls out of a slaughtered animal onto the ground (or someplace not in the animal)? ![]() Chiss Boy 12:34, 4 March 2007 (UTC)
Chiss Boy 12:34, 4 March 2007 (UTC)
4 chains vs. 2 chains
I read some time ago that while in "higher" animals including humans, 2 strands of alpha-hemoglobin combine with 2 strands of beta-hemoglobin, in "lesser" animals, the hemoglobin consists only of 2 alpha-strands (enclosing, as usual, an iron atom). Unfortunately, I had no luck finding more info on the Internet - could somebody who knows about this please elaborate? Aragorn2 11:50, 23 Oct 2004 (UTC)
If you go "low" enough, hemoglobin acts on nitrous oxide rather than oxygen [1]. A little "higher" and animals have large molecular weight hemoglobins that are carried in the plasma. Move on up to birds and mammals, and there's small molecular weight hemoglobins that are packaged in red blood cells [2]. The respiratory protein of "lower" animals certainly isn't always simpler (snail hemocyanin, for example, has 10 subunits). I don't know of any animals that have only two alpha-strands as a functional hemoglobin, and alpha-beta dimers are ineffective oxygen carriers, so something's been lost since your read it, or the missing info is in a veterinary source somewhere. - Nunh-huh 14:08, 23 Oct 2004 (UTC)
Thanks for the links. I had read about the worm before, but from this text, it's not obvious whether the worm has dimer or tetramer (or even monomer?) hemoglobin. After another Google search, I found this:
and a possible scenario for hemoglobin evolution on this page:
So the lamprey has alpha-alpha-dimer hemoglobin which splits apart when oxygenated, which is what I was referring to.
Got it now.. looks like:
- hagfish: monomeric hemoglobin in most species, some have hgb that is dimeric when deoxygenated and monomeric when oxygenated
- lamprey: monomeric when oxygenated, dimeric or tetrameric when deoxygenated
- sharks: dimeric when oxygenated (α1β1); tetrameric when deoxygenated
- vertebrates: α2β2 dimers
That was a very interesting question! we ought to put a chart or a synopsis in the article. - Nunh-huh 03:52, 24 Oct 2004 (UTC)
There are variety of hemoglobin predecessors, many of which have different names. Such as Erythrocruorin in worms and myoglobin which is a monomer and has no arrogating ability. Erythrocruorin should get an article for it self, I can easily scrounge up enough information and generate image of the protein from pubmed, but I lack the time.--BerserkerBen 22:58, 31 Jan 2005 (UTC)
Human Hemoglobin Levels
I don't know much about the chemistry of hemoglobin and the like, but a section detailing how and why hemoglobin levels are measured in humans. A table or something illustrating the distribution and ideal hemoglobin level would also be great. My doctor just called me and all he said was "14,7" and I have absolutly no clue what that means. Google will answer me in minutes, but still, I was hoping Wikipedia would have it. --Crucible Guardian 6 July 2005 01:29 (UTC)
- Me too! I just found out mine is 17.4 which is higher than normal, but again this article doesn't explain the signifance of higher levels of hemoglobin.--77.224.22.177 (talk) 20:59, 31 May 2009 (UTC)
Salt bridges
What does salt bridges mean in this context? Not the same as the linked article, anyhow. / Habj 18:28, 20 October 2005 (UTC)
- I suspect it means ionic bonds but since I don't know, I just remove it and rephrase the sentence. / Habj 19:07, 21 October 2005 (UTC)
- That's correct. See here / Djdaedalus 14:50, 24 October 2005 (UTC)
hemoglobin and aging
Sometime in the past I read that in humans, oxygen transport decreases by 1% per year after the age of 20, which I can believe, being now 65. Is there information on the aging process and oxygen transport that could be summarized in a short paragraph?
- If someone supplies the references, yes (see WP:CITE). A quick Google will probably yield some results. JFW | T@lk 08:51, 2 December 2005 (UTC)
Consistency
This article goes back and forth between hemoglobin and haemoglobin. Pick one and stick with it, but mention both in the first sentence. I believe the hemoglobin spelling is more universal throughout medical literature, but I don't know about common usage outside the US. Hichris 17:39, 16 December 2005 (UTC)
- It was pretty uniform at "hemoglobin" until this was changed [3]. My literature might have been american, but I think hemoglobin is the one to choose. / Habj 03:46, 17 December 2005 (UTC)
- I would prefer hæmoglobin! That is the traditional spelling. Failing that, then haemoglobin. It's only in North America that the "simplified" version is used. EuroSong talk 09:30, 2 November 2006 (UTC)
- I guess haemoglobin would be better as it is the traditional spellingVik4989 06:25, 5 August 2007 (UTC)
- I would prefer hæmoglobin! That is the traditional spelling. Failing that, then haemoglobin. It's only in North America that the "simplified" version is used. EuroSong talk 09:30, 2 November 2006 (UTC)
Yeah we should really be using haemoglobin. —Preceding unsigned comment added by 194.81.199.59 (talk) 16:44, 11 February 2008 (UTC)
Hemoglobin is the spelling far more commonly used in scientific literature. Searching Medline turns up ~76,000 hits for "hemoglobin" and under 20,000 for "haemoglobin." —Preceding unsigned comment added by 128.237.250.128 (talk) 20:32, 24 April 2008 (UTC)
Feature article anyone?
Anyone think this artilce shoukld be nominated as a feature article? If not why?--BerserkerBen 07:31, 31 December 2005 (UTC)
- It is not ready. Saravask 20:01, 31 December 2005 (UTC)
- What needs to be done to make it ready? --BerserkerBen 20:06, 17 January 2006 (UTC)
I can think of some points. Firstly, the spelling is inconsistent (BE/AE). There is no history section; we don't learn who discovered it, when it was first sentenced, linked and cloned, why Linus Pauling won the Nobel Prize in relation to it, why the Bohr effect is called like that, etc etc. In short, more context. Classic references are very useful. JFW | T@lk 21:28, 17 January 2006 (UTC)
- Great can we list those things up on top of the discussion board as things needed for improvemnt?--BerserkerBen 23:59, 17 January 2006 (UTC)
Question
So pretty much hemoglobin is a key to a healthy heartbeat.
- Uhh, that is only partially true. For a healthy heartbeat the hemoglobin-containing blood also needs to be delivered to the myocardium, which requires a functioning coronary circulation unimpaired by atherosclerosis, as well as a myocardium that is not remodelled as a result of excessive strain, poor metabolism or hereditary muscle abnormalities. There is really quite a lot to a healthy heartbeat... JFW | T@lk 17:17, 8 February 2006 (UTC)
What?
This process also produces one molecule of carbon monoxide for every molecule of heme degraded [4]; this is one of the few natural sources of carbon monoxide production in the human body, and is responsible for the normal blood levels of carbon monoxide even in people breathing pure air.
Am I the only one that finds this a bit wierd, saying, "Pure" air. What exactly is "unpure" air. I think this should be reworded but I'm not sure how. --RobertDahlstrom 18:32, 9 November 2006 (UTC)
- That's a good question. I can only guess that "pure air" refers to the "normal" atmospheric mix of gasses, of which carbon monoxide composes only trace amounts. – ClockworkSoul 19:04, 9 November 2006 (UTC)
opening part of article not clear
"Hemoglobin or haemoglobin (frequently abbreviated as Hb or Hgb) is the iron-containing oxygen-transport metalloprotein in the red cells of the blood in mammals and other animals; in mammals the protein makes up about 97% of the red cell’s dry content, and around 35%, including water."
35% .. ??
Jdruiter 23:32, 28 January 2007 (UTC)
- RBCs are about 66% water. SBHarris 00:49, 29 January 2007 (UTC)
I was in the ER two times yesterday with my sister for nausea.Her hemoglobin was 7.9.today she still has a bad lingering headache. Could this headache be caused from her low hemoglobin? She has been sick for years. She's has really bad migraines, has had her gallbladder and half her intestines taken out,and she can't digest her food which makes her throw up.she's had to have a blood transfusion 5 months ago, because her hemoglobin was 6.1.Can anyone answer my question about if low hemoglobin can cause her to have this lingering headache?(she only weighs 80 lbs and is 56 yrs.old.)(she said this headache is not like her migraines.)
Thank you,
Sincerley
Judy Vasilis
Origin
Some comment on the evolutionary origin of the haemoglobins would be welcome. For example, the similarities of the porphyrin cores with chlorophyll. Is there a common ancestor to both? Fig 10:27, 26 February 2007 (UTC)
Someone I know said that the only difference between the hemoglobin molecule and chlorophyll is the nitrogen in chlorophyll and the iron in hemoglobin. I think its a gross simplification and I am by no means an expert, but it got me curious about the similarities and differences between the two. --黒雲 user:Qaddosh 02:35, 17 August 2007 (UTC)
2 images for R to T transformation?
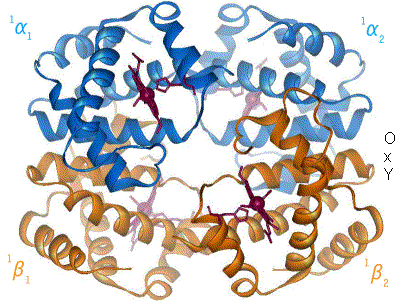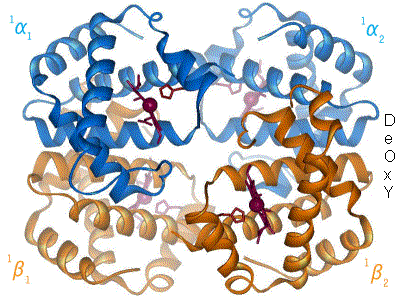
I have undone a recent addition of a second image to show the R to T transformation. [4] The reasoning is that, although the second image does show all 4 chains and you could argue that two images are better than one, the text was being crushed up along the left hand side (as the second image is so big) and the sigmoid curve image (which is already some distance from the text referring to it) was being pushed further down the page. If someone who is better at image placement than I am can resolve these difficulties I would have no problem with the 2 images. Thanks. Mmoneypenny 21:55, 28 April 2007 (UTC)
- Remember, we're now all being asked to take out the "px" setting in images, and allow our personal readers to set that for us, so all images in an article come out the same size. If you've got yours set too high (look at your "my preferences") like 300 px vs. the recommended 180, then a lot of pics which support 300 will crowd your screen more than you'd like. Also, the images that don't have enough detail to be 300 px wide or long will come out odd size, and small by comparison with 300. But fix that on YOUR end, not in the wiki.
I'll do my best to make two pics of hemoglobin at 180px come out without shoving the sigmoid curve too far down. But we really need two pics of hemoglobin motion at least as much as the sigmoid curve. SBHarris 22:09, 28 April 2007 (UTC)
- Remember, we're now all being asked to take out the "px" setting in images, and allow our personal readers to set that for us, so all images in an article come out the same size. If you've got yours set too high (look at your "my preferences") like 300 px vs. the recommended 180, then a lot of pics which support 300 will crowd your screen more than you'd like. Also, the images that don't have enough detail to be 300 px wide or long will come out odd size, and small by comparison with 300. But fix that on YOUR end, not in the wiki.
- Later. Okay, I've done my best to make it all fit, by putting the moving O2 binding pics side by side. Constraints are that the one image (the old one) requires the command "frame" to move at all. And I cannot get it to any size other than 300 px, no matter what I order. The other image is perfectly well behaved. I moved the sigmoid curve up, and reduced its size do it fits better along side the text it references. I don't know what 180px readers will do to this, for those people who insist on removing all "px" image commands. But this is a case where I think it's justified to leave all the extra commands in. Wiki policy allows this, where you just can't get it to look good any other way. SBHarris 22:43, 28 April 2007 (UTC)
- I appreciate that we are removing the px settings. I also appreciate that we can set the thumbnail size in our preferences (this is set at 180 for me) but I hope you appreciate that a framed image will always be displayed at its full size and the px command will not affect it, so this is not something I can change at my end. (Regardless of this and slightly unrelated, most people browse wikipedia without being logged in and they cannot set a preference.) Lastly, do we really really need two pics of hemoglobin motion? IMO we are trying to make people appreciate that there is a motion (as explained in the text) and the first pic shows this quite well, the second pic adds the other 2 chains but removes the oxygen... Surely if people read the text and appreciate that Hb consists of four chains and (as the first pic states) we are only showing one, that is enough? I see that the two images are now at the top of the subheading with some text shoved in between them, this still IMO looks bad. The result is an extra pic but a messy article. When I've got a bit more time I'll see if I can play around with the pics a bit more. All the best.Mmoneypenny 08:37, 29 April 2007 (UTC)
- In my reader, when I set the one nonframe illustration to 300px, there's no room for any text, so it looks pretty good. I had no idea others were having a problem.
I haven't found a way to keep text out of a section that you only want to put illustration boxes in, no matter the size. I finally put in enough equalsign spacer to keep text out of there no matter what the illustration size is. What do you think of the look now?
The main reason I'm trying to keep the old illustration in is not because I made it or had anything to do with it. But it shows the motion of the porphyrin ring-- how it moves to react with the O2 and tugs on its peptide chain by doing so, far better than the other. I can almost see all the details of how the molecule works here, and would far rather remove the latest version, if I had to pick one. SBHarris 05:38, 30 April 2007 (UTC)
- I asked for help using the {{helpme}}tag on my talk page and another user has modified the paragraph. Okay, I know now the two illustrations are distant but... how about rewriting the paragraph and the image blurbs to match the new setup AND we can place the image you prefer at the top and the other one further down. Would do this myself but am slightly busy at the mo (aren't we all?) Whaddya think?Mmoneypenny 20:35, 30 April 2007 (UTC)
- Unfortunately, the "help" didn't help at all. Either of us could simply have done what was done, which was stick one of the binding pics way down in the article where it didnt' have anything to do with the text. But they both need to be up near each other, and still give the sigmoid curve some room to be were it needs to be. And unfortunately the sigmoid discussion needs to be right after the binding discussion, in order to make sense. So the problem is not solved. SBHarris 21:48, 30 April 2007 (UTC)
- I asked for help using the {{helpme}}tag on my talk page and another user has modified the paragraph. Okay, I know now the two illustrations are distant but... how about rewriting the paragraph and the image blurbs to match the new setup AND we can place the image you prefer at the top and the other one further down. Would do this myself but am slightly busy at the mo (aren't we all?) Whaddya think?Mmoneypenny 20:35, 30 April 2007 (UTC)
- In my reader, when I set the one nonframe illustration to 300px, there's no room for any text, so it looks pretty good. I had no idea others were having a problem.
(cough)I was the one that made taht animation if you need my help or anthing? (cough) --BerserkerBen 23:38, 30 April 2007 (UTC)
- We need animations which don't force the reader to see them at 300px because they've been done via FRAME. I don't know if that's possible, but it must be, because the first animation does it. But I can't make the second one do it, and still move. The problem is that having one at 300px and the other at the default size of whatever the viewer's reader is, is bound to screw up the text, if the two are near each othr. Which it would be nice if they were. But nobody's been able to get them side by side and keep everybody happy. SBHarris 02:56, 3 May 2007 (UTC)
- Well I guess it some flaw with my gif animator if you want the orginal frames so you can animate it please ask.--BerserkerBen 03:49, 3 May 2007 (UTC)
Oxidation state
The section about the oxidation state of iron in oxyhaemoglobin is wrong. It is now known that it is low-spin Fe(II). See Greenwood and Earshaw, Chemistry of the Elements 2nd edition, pp 1099-1100. The key point is that in deoxy-Hb the high-spin Fe(II) is too large to enter the plane of the porphirin ring (Figure 25.7) but when oxygen binds the low-spin Fe(II) enters the plane as it is small enough. The paramagnetism of O2 is destroyed because the Fe-O2 angle is 120o, so the pi-star orbitals are no longer doubly degenerate.
I'm not a biochemist, so I hesitate to edit the article myself.Petergans 09:56, 11 May 2007 (UTC)
- I made the same mistake in originally fleshing out this section. Apparently the old classic crystal field theory view of the iron pi-star orbitals being forced to give up their electrons into low spin pairs when O2 enters the picture, is wrong. Fe(III) when present in free hemoglobin won't bind oxygen, but oddly enough X-ray spectrocopy and IR show that iron in oxygenated hemoglobin actually is Fe(III), and the O2 bond had elongated enough that it's really a bond with order 3/2, i.e. it is superoxide .O2-. So the (surprising) fact is that O2 binding to hemoglobin involves a (temporary) partial oxidation of iron to Fe(III) and reduction of O2 to .O2-. Basically about one electron gets temporarily transferred in a charge-transfer complex (of course you realized that electrons often don't get transfered exactly, but only approximately, since these bonds have some covalent character). Anyway, that's the modern picture. SBHarris 18:40, 11 February 2008 (UTC)
-SBHarris, do you have reference for that? —Preceding unsigned comment added by 216.80.44.45 (talk) 02:06, 24 October 2008 (UTC)
- Yes, which I'll add: http://www.ul.ie/~childsp/CinA/Issue65/TOC28_Haemoglobin.htm. Basically, all the evidence points to oxyhemoglobin being a complex of low-spin Fe(III) and superoxide. The oxygen bond order is 1.6 by IR, meaning it's been nearly reduced to superoxide, and the whole complex is diamagnetic (no net spin) which can only happen if the single unpaired electron on O2.- pairs with the single unpaired electron in low spin Fe(III) by a long distance (anti)ferromagnetic interaction, giving the whole complex no net spin. The standard idea of a triplet neutral O2 (paramagnetic) paired with low spin Fe(II) (diamagnetic) would produce a net paramagnetic HbO2, and HbO2 isn't paramagnetic. To get a diamagnetic HbO2 with low-spin Fe(II) you have to have diamagnetic O2, which means singlet oxygen. This higher energy solution is apparently not chosen by nature. Extraction of an additional electron from iron allows for a smaller Fe atom, and that allows it back into the plane of the porphyrin ring, which needs to happen for the allosteric interaction. I'll do some more explaining in the article. SBHarris 04:00, 28 October 2008 (UTC)
hemoglobin or haemoglobin
Is it called hemoglobin or haemoglobin. The title says hemoglobin but in the opening line of the article it says haemoglobin. Which one should be changed? Ziphon 02:47, 29 May 2007 (UTC)
- Hi. There's no consensus on the "correct" spelling. However, we should use 1 spelling throughout the entire article for consistency, and mention alternate spellings only once in the article's introduction. Greets, A. Rad 16:29, 30 May 2007 (UTC)
- Considering that Funk spelled the original Template:De icon word "Hämoglobin" in 1851, I'd suggest that has seniority. The "Haemo" spelling is clearly a more faithful transliteration than "Hemo", both are widely used so we need a redirect anyhow. Why not use the original for the title and make both transliterations redirects?LeadSongDog (talk) 20:04, 18 April 2008 (UTC)
- The remainder of the article employs the American flavor of English (personally, I prefer grape, but I'm rather odd), so tradition and WP:ENGVAR agree that we should stick with that unless somebody converts the entire article to another flavor, an act which is usually considered to be very bad form. It seems to me, then, that hemoglobin (and fetus) are most appropriate. – ClockworkSoul 21:36, 18 April 2008 (UTC)
The original article was written in British English, and according to wikipedia rules, any form of english that is widely accepted is acceptable, so long that this was the original form the article took. Applying this, given the original article (tracking history back as far as i can) has the spelling 'haemoglobin', I move for this to be reinstated. 86.7.204.43 (talk) 21:30, 30 August 2008 (UTC)
- True, but the article was created at the American spelling, Hemoglobin, and a spelling change would require a move. It's hard to say whether it's the article creator's choice of location or primary spelling method within the article that should be honored (honoured?) first. It's kind of an odd situation, and I'm inclined to say leave well enough alone. -- Vary | Talk 18:42, 31 August 2008 (UTC)
Mycoglobin
Is mycoglobin not a form of hemoglobin? --Filll 19:10, 8 June 2007 (UTC)
- Do you mean Myoglobin? I'm not sure what mycoglobin is (for funguses maybe?). Myoglobin is the form of hemoglobin for transportation of oxygen in muscles fiber. Orangemarlin 20:10, 8 June 2007 (UTC)
Personal Blog
Im too busy to fix but this link at bottom of this article links to a personal blog unrelated to subject.
Information on Haemoglobin
CRH
- I don't see it. I think its been removed. LostLucidity (talk) 19:55, 3 April 2008 (UTC)
please help
can anybody please mail me or refer me to any link that would help me understand the biochemical process of oxidation of myoglobin n haemoglobin along with their protein tertiary n quartenary structures???Vik4989 06:25, 5 August 2007 (UTC)
Empirical chemical formula doesn't match molecular weight
The empirical formula C738H1166N812O203S2Fe gives a molecular weight of: 738*12+1166+812*14+203*16+2*32+56 = 24758 dalton. This does not match the quoted molecular weight of roughly 17,000 dalton for each subunit, nor does it match a total molecular weight of roughly 68,000. —Preceding unsigned comment added by Dan Gluck (talk • contribs) 06:49, 18 September 2007 (UTC)
- Good pickup. Something's wrong. To start, the formular given is obviously just for one subunit, since there's only one iron! We'll look it up and fix something. SBHarris 18:00, 11 February 2008 (UTC)
- Well, I need help. The empirical chem formula (C2952H4664N812O832S8Fe4), was added here [5] without a cite, Sept 5, 2006. That was this author's last addition to Wikipedia. In addition, it's a formula for a hemoglobin of about 65,250 d, or so, with 4 irons. I've gotten various figures of 64,000 to 67,000 (what the article currently says) for this molecule. Along the line since, somebody cut the above formula down (dividing C, H, S, and F by 4, leaving N alone, and changing O from 832 to 204!). That's CLEARLY wrong. And that's the number that has survived on Wikipedia, giving everybody the wrong empirical formula for hemoglobin, for a year and a half! It's now all over google! Shameful. I've just deleted it until we can get this right. SBHarris 06:56, 3 March 2008 (UTC)
- Good pickup. Something's wrong. To start, the formular given is obviously just for one subunit, since there's only one iron! We'll look it up and fix something. SBHarris 18:00, 11 February 2008 (UTC)
- It is best to leave it off, empirical formulas for proteins are Meaningless - whether it has 738 carbons or 400 carbons doesn't tell you anything about what or how it works. Knowing the approximate size and number of amino acids does. Hichris (talk) 16:15, 9 April 2008 (UTC)
Globin genes
I come from more of a genetics background and knew hemoglobin in the context of the alpha globin locus, the beta globin locus and locus control region, the genes, etc. It took me forever to get my bearings here because "variants" refer to different combinations of protein subunits, not different protein subunits themselves. I now realize that α,β,etc. are the protein subunits, but I don't think this was explained very well and the protein subunits themselves definitely aren't talked about much. For now I'll just add links under "See also" to the genes, since I'm not sure on a good consistent nomenclature for the article. Forluvoft 06:52, 3 November 2007 (UTC)
- Wups, "variants" is used in BOTH senses: not only to different tetramers, but also (and mainly) to the many, many polymorphisms in the protein chains themselves (like sickle cell, but producing no clinical problems, even when homozygous). This needs a fix, to differentiate. We at one time had an actual article on Hemoglobin variants which I started, and which somebody took down and redirected to hemoglobin! I put it up at first for this very reason. Damn deletionists. This is a good example of how they harm the encyclopedia. There certainly isn't room enough in this article to discuss all of these sequence variants, only to discuss the tetramer variants. The gene variant article subarticle which had't grown up yet, and was killed in its cradle. Arghh. If you complain about this kind of thing, some admin who isn't interested in the subject will say that "wikipedia isn't the humane society for lost information." Well, that's wrong, or should be. SBHarris 18:06, 11 February 2008 (UTC)
History and art section
This section seems utterly pointless. First, there's no history in the "History" part - just some unsupported and irrelevant speculation about the planet Mars and "the ancients". Second, the "art" section cites one piece of sculpture (this is, I suspect, a piece of self-promotion by the artist, or possibly put in by a friend - there's a similarly irrelevant paragraph in the alpha-helix article). Third, this is an article about molecular biology, not about art. The entire section should be removed.
- Well, it's supposed to be an article about hemoglobin in all of its aspects in human knowledge, including history and art. The molecular bio of hemoglobin (about which nothing at all was known half a century ago) would be a subarticle (or several of them, one for gene control, one for gene allelic variation) leaving a summary here (not done yet). The fact that the art and culture section hasn't yet been fleshed out and is under construction, only means this is wikipedia. You don't get the way the place works. Half the article looked like that a while ago.
Bottom line, if you have info about the sections you complain are lacking info or balance, contribute it! When the article gets too full of mol bio, those parts will get spun off to subarticle, leaving good summaries here. Books have been written on every aspect of this, as you know, and only a fraction of that is here. More of it will be. SBHarris 18:14, 11 February 2008 (UTC)
"create a strong pocket for the heme group"
what chemical interactions are responsible for the strong binding of the heme group and globin structure? Hydrophobic bonds? Hydrogen bonding? electrostatic? Thanks. —Preceding unsigned comment added by 75.43.198.198 (talk) 21:41, 17 February 2008 (UTC)
Suggestion for improvement
I would contribute this myself, but I'm not a very good writer. look at this information: http://sickle.bwh.harvard.edu/hbsynthesis.html much that is important here, isn't listen in the article. especially the # of globin molecules. it isn't clear that each heme binds to one globin, also the changeover from fetal Y globin to B in adult should be explained, and as an example elaborate on how some patients exhibit no symptoms until they start to make the change over and they realized their B is defective genetically (thallassemia)
cheers, purpleidea (talk) 22:55, 4 March 2008 (UTC)
Too much?
The "role in disease" section lists several diseases of "too little" or "broken" hemoglobin. What is it when you have too much hemoglobin? Polycythemia? Something else? WhatamIdoing (talk) 03:55, 22 August 2008 (UTC)
- In practice, polycythemia is the only way this happens. It's not so much the having of too much hemoglobin that is pathological, as having too much fraction of blood made of cells (packed cell volume or "hematocrit" too high). These amount to (almost) the same thing because concentration of hemoglobin in cells can't vary much (it can been lower than normal, but not much higher, and cannot vary enough to affect the major problem of too many cells). In theory your total cell mass per volume could be high enough to make you ill (clots) but due to giant cells with low hemoglobin, everything but packed cell volume would be normal. However, in practice you don't see this. And you can't see high hemoglobins without also high PCVs because of the fact that hemoglobin is already at near the max concentration in normal cells anyway, so the only way to increase it in blood significantly is to incease the total cell-volume fraction in blood. Sorry that's not clearer, but it's written on the fly. SBHarris 21:38, 30 August 2008 (UTC)
- Thanks. I added it and hope we can find a good source for it. WhatamIdoing (talk) 02:44, 31 August 2008 (UTC)
Correction or vandalism?
Can someone double check this edit, which changes a single number? WhatamIdoing (talk) 23:25, 11 December 2008 (UTC)
Advertising
About this kind of edit:
Wikipedia is not a platform for promoting new products. Congrats on getting FDA approval, and I hope all goes well -- but frankly, the fact that hgb can be measured with this device is not really important. Language like "new" and "only way" is a violation of WP:PEACOCK. We don't care what company makes it, we don't care when it was approved in a single country -- it's just not important. If we wanted to include this information, it would be very dull: "Hemoglobin is normally measured through a blood draw, but a noninvasive option is also available." No company name, no press-release style, nothing that smacks of advertising.
Finally, this information off-topic for the section it keeps reappearing in, which is about diagnostic uses (e.g., figuring out how well diabetes is being controlled), not diagnostic devices.
Please do not keep making the same mistakes. WhatamIdoing (talk) 22:51, 23 December 2008 (UTC)
Reference range
I do not know much about the chemistry of hemoglobin and the like, but a section detailing how and why hemoglobin levels are measured in humans. A table or something illustrating the distribution and ideal hemoglobin level would also be great. My doctor just called me and all he said was 7 and I have absolutly no clue what that means. Google will answer me in minutes, but still, I was hoping Wikipedia would have it. --Crucible Guardian 6 July 2005 01:29 (UTC) —Preceding unsigned comment added by Ohiohealthquiz (talk • contribs)
- You'll find your answer at this page, but it could be added to this article (or a link provided to the other one) if someone wanted to do that. WhatamIdoing (talk) 03:01, 31 December 2008 (UTC)
Discovery?
I don't know about this edit. If we've got a source that says 1851, then why are we replacing it with one that asserts a 1940 discovery? WhatamIdoing (talk) 20:11, 8 January 2009 (UTC)
