Cuprate

Cuprates (from Latin cuprum meaning copper) are chemical compounds containing copper anion. Cuprates have been known for centuries and are widely used in inorganic and organic chemistry. However, interest in them has significantly increased since 1986 after the discovery of high-temperature superconductivity in a lanthanum barium copper oxide by Georg Bednorz and Karl Müller.[1] More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001,[2] and Bednorz and Müller were awarded the Nobel Prize in Physics only a year after their discovery.[3] From 1986 to 2008, almost all known high temperature superconductors were cuprate superconductors. By 2008, the highest confirmed, ambient-pressure, superconducting transition temperature (Tc) is the value of 135 K achieved in a layered cuprate HgBa2Ca2Cu3Ox in 1993.[4][5]
Terminology
When copper compounds, which is involved in a larger coordination complex, has an overall negative charge, then it is referred to as "cuprate", for example, amminepentachlorocuprate(II) [NH3CuCl5]2–. However, tetraamminedichlorocopper(II) (NH3)4CuCl2 has a neutral charge and therefore is not a cuprate ion.[6]
Inorganic cuprates

Cuprate anions form complexes with negatively charged ligands such as cyanide, hydroxide, or halides. Typical representatives of these complexes are tetracyanocuprate(I), [Cu(CN)4]3–, tetrachlorocuprate(II) [CuCl4]2– and hexahydroxocuprate(II) [Cu(OH)6]4–. There are also rare copper(III) and copper(IV) complexes such as the hexafluorocuprate(III) [CuF6]3– or hexafluorocuprate(IV) [CuF6]2–, which are strong oxidizing agents. Whereas tetracyanocuprate(I) [Cu(CN)4]3- is colorless,[7] most other copper(I) complexes are red-brown; copper(II) complexes have intense turquoise blue color while copper(III) and copper(IV) complexes are orange-red.[8] Dilithium tetrachlorocuprate (Li2CuCl4) is an effective catalyst for the couplings of alkyl halides in the Grignard reaction. It is prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in tetrahydrofuran.[9]
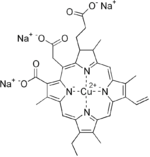
Organic cuprates

Cuprates play important role in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide Cu2C2, was synthesized by R.C. Bottger in 1859.[10]
Organic cuprates usually contain an R2Cu moiety, where R is a carbon containing unit. They are rather reactive towards oxygen and water forming copper(I) oxide; they are generally insoluble in inert solvents, tend to be thermally unstable and are difficult to handle. Nevertheless, organocopper reagents are frequently used in organic chemistry as alkylating reagents prepared in situ in an inert environment.[11] The electronegativity of copper is much higher than its neighbor in the group 12 elements, zinc, suggesting less nucleophilicity for carbon.
 |
 |
 |
Ionic cuprates and superconductivity
Ionic cuprate contain copper in a rare oxidation state 3+, such as CuO–
2. Typical examples are yttrium barium copper oxide (YBCO) and bismuth strontium calcium copper oxide (BSCCO, see top figure for structure), which are the most popular high-temperature superconducting materials. Although such oxides were known for decades, only in 1986, superconductivity was discovered by Georg Bednorz and Karl Müller in a lanthanum barium copper oxide. The transition temperature in their material BaxLa5-xCu5O5(3–y) was "only" Tc = 35 K, this finding stimulated worldwide research on the synthesis and superconductivity in cuprates. Next year, the Tc value was increased to 90 K in the famous YBa2Cu3O7 (YBCO) cuprate.[13] That was a breakthrough achievement because the new superconductors could be cooled by relatively cheap liquid nitrogen rather than expensive liquid helium. Soon after, superconductivity was found in bismuth strontium calcium copper oxide (BSCCO or Bi2Sr2CanCun+1O2n+6-d) with Tc = 95–107 K depending on the n value. Thallium barium calcium copper oxide (TBCCO, TlmBa2Can−1CunO2n+m+2+δ) was the next class of high-Tc cuprate superconductors with Tc = 127 K observed in
Tl2Ba2Ca2Cu3O10 (TBCCO-2223) in 1988.[14]
In 1993, the transition temperature reached 135 K in a layered cuprate HgBa2Ca2Cu3Ox which by 2009 remains the highest confirmed ambient-pressure Tc value. Whereas the low-temperature superconductivity in most conventional materials was well explained by the BCS theory (Nobel Prize in Physics in 1972), the mechanism of the high-temperature superconductivity in cuprates remains unsolved.[4][5]
More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001,[2] and Bednorz and Müller were awarded the Nobel Prize in Physics in 1987.[3] BSCCO superconductors already have large-scale applications. For example, tens of kilometers of BSCCO-2223 electrical cables are being used in the Large Hadron Collider – the world's largest and highest-energy particle accelerator.[15]
-
The Meissner effect demonstrated by levitating a magnet above a cuprate superconductor, which is cooled by liquid nitrogen.
-
A one-millimeter piece of BSCCO.
-
For practical applications, BSCCO is compressed with silver metal into a tape via the PIT process
See also
References
- ^ J.G. Bednorz and K.A. Mueller (1986). "Possible high TC superconductivity in the Ba-La-Cu-O system". Z. Phys. B64 (2): 189–193. doi:10.1007/BF01303701.
- ^ a b Mark Buchanan (2001). "Mind the pseudogap". Nature. 409: 8. doi:10.1038/35051238.
- ^ a b Nobel prize autobiography
- ^ a b Lee, Patrick A (2008). "From high temperature superconductivity to quantum spin liquid: progress in strong correlation physics". Reports on Progress in Physics. 71: 012501. doi:10.1088/0034-4885/71/1/012501.
- ^ a b Chu, C. W. (1993). "Superconductivity above 150 K in HgBa2Ca2Cu3O8+δ at high pressures". Nature. 365: 323. doi:10.1038/365323a0.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help) - ^ G. Burton (2000). Chemical ideas. Heinemann. p. 268. ISBN 0435631209.
- ^ Thomas J. Bruno, Paris D. N. Svoronos (2004). Handbook of basic tables for chemical analysis. CRC Press. p. 543. ISBN 084931573.
{{cite book}}: Check|isbn=value: length (help) - ^ Egon Wiberg, Nils Wiberg, Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1252–1264. ISBN 0123526515.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Atta-ur-Rahman (2002). Bioactive natural products. Elsevier. pp. 73, 81, 83. ISBN 0444510044.
- ^ R. C. Böttger (1859). Annalen. 109: 351.
{{cite journal}}: Missing or empty|title=(help) - ^ Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1891389041.
- ^ Lorenzen, Nis Peter (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt)2}(CuPh2)]2". Angewandte Chemie International Edition in English. 29: 300. doi:10.1002/anie.199003001.
{{cite journal}}:|first2=missing|last2=(help) - ^ K. M. Wu; et al. (1987). "Superconductivity at 93 K in a new mixed-phase Yb-Ba-Cu-O compound system at ambient pressure". Phys. Rev. Lett. 58 (9): 908. doi:10.1103/PhysRevLett.58.908. PMID 10035069.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Sheng, Z. Z. (1988). "Bulk superconductivity at 120 K in the Tl–Ca/Ba–Cu–O system". Nature. 332: 138–139. doi:10.1038/332138a0.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "HTS materials for LHC current leads". CERN.



