Solar cell
A solar cell, or photovoltaic cell, is a not specifically a "p-n junction". This is in fact a specific example of a photovoltaic device characteristic of the First Generation of solar cells, including Si wafer devices. More generally a solar cell is a semiconductor device that converts photons (light) into electricity. Fundamentally, the device needs to fulfill only two functions: 1. Photogeneration of charger carriers (electrons and holes) in a light-absorbing material, and 2. Separation of the charge carriers, preferably to a conductive contact that will transmit the electricity. This conversion is called the photovoltaic effect, and the field of research related to solar cells is known as photovoltaics.

The most common configuration of this device, the first generation photovoltaic, consists of a large-area, single layer p-n junction diode, which in the presence of sunlight is capable of generating usable electrical energy. These cells are typically made using a silicon p-n junction. However, successive generations of photovoltaic cells are currently being developed that may improve the photoconversion efficiency for future photovoltaics. The second generation of photovoltaic materials is based on multiple layers of p-n junction diodes. Each layer is designed to absorb a successively longer wavelength of light (lower energy), absorbing more of the solar spectrum and increasing the amount of electrical energy collected. The third generation of photovoltaics is very different from the other two, and is broadly defined as a semiconductor device which does not rely on a traditional p-n junction to separate photogenerated charge carriers. These new devices include dye sensitzed cells, organic polymer cells, and quantum dot solar cells.
Solar cells have many applications. They are particularly well suited to, and historically used in, situations where electrical power from the grid is unavailable, such as in remote area power systems, Earth orbiting satellites, handheld calculators, remote radiotelephones and water pumping applications. Solar cells (in the form of modules or solar panels) on building roofs can be connected through an inverter to the electricity grid in a net metering arrangement.
Etymology
The term "photovoltaic" comes from the Greek phos meaning "light", and the name of the Italian physicist Volta, after whom the volt (and consequently voltage) are named. It means literally of light and electricity.
History
Main article: Timeline of solar cells
The photovoltaic effect was first recognised in 1839 by French physicist Alexandre-Edmond Becquerel. However it was not until 1883 that the first solar cell was built, by Charles Fritts, who coated the semiconductor selenium with an extremely thin layer of gold to form the junctions. The device was only around 1% efficient. Russell Ohl patented the modern solar cell in 1946 (US2402662, "Light sensitive device"). Sven Ason Berglund had a prior patent concerning methods of increasing the capacity of photosensitive cells.
Simple explanation
- Rays of sunlight hit the solar panel and are absorbed by semiconducting materials, such as silicon
- Electrons are knocked loose from their atoms, allowing them to flow through the material to produce electricity
- An array of solar panels converts solar energy into a usable amount of direct current electricity
Additionally, for everyday use:
- The DC current enters an inverter
- The inverter turns DC electricity into 120 or 240-volt AC (alternating current) electricity needed for home appliances
- The AC power enters the utility panel in the house
- The electricity is then distributed to appliances or lights in the house
Materials and efficiency
Various materials are being investigated for solar cells. Peformance in the two main criteria, efficiency and costs, varies greatly.
Efficiency is the ratio of the electric power output to the light power input. At solar noon on a clear March or September equinox day, the solar radiation at the equator is about 1000 W/m². So a 10% efficient module of 1 square meter has a power output of about 100 W. Solar cell efficiencies vary from 6% for amorphous silicon-based solar cells to 30% or higher with multiple-junction research lab cells. However the highest efficiency cells may not be the most economical - a 30% efficient multijunction cell based on exotic materials such as gallium arsenide or indium selenide might cost say 100x as much as an 8% efficient amorphous silicon cell, while only delivering 4x the electrical power.
A common method used to express economic costs of electricity generating systems is to calculate a price per delivered kilowatt-hour (kWh). The solar cell efficiency in combination with the available irradiation has a major influence on the costs, but generally speaking the overall system efficiency is important. To make actual use of the solar generated energy, the electricity is most often fed into the electricity grid using inverters (grid-connected PV systems); in stand alone systems, batteries are used to store the electricity that is not needed immediately. Using the present (2005) commercially available solar cells and system technology leads to system efficiencies between 5 and 15%. Electricity generation costs range from around 50 eurocents/kWh (0.60 US$/kWh) (middle of Europe) down to around 25 eurocents/kWh (0.30 US$/kWh) in regions of high solar irradiation. Since, in grid-connected applications, this electricity is generally fed into the grid on the customer's side of the meter, it can be compared to prevailing retail electric pricing, which varies from between 0.04 and 0.50 US$/kWh worldwide.
By far the most common material for solar cells (and all other semiconductor devices) is crystalline silicon. Crystalline silicon solar cells come in three primary categories:
- Single crystal or monocrystalline wafers made using the Czochralski process. Most commercial monocrystalline cells have efficiencies on the order of 16–17%; the SunPower cells have higher efficiencies, around 20%. Single-crystal cells tend to be expensive, and because they are cut from cylindrical ingots, they cannot completely cover a module without a substantial waste of refined silicon. Most monocrystalline panels have uncovered gaps at the corners of four cells. Sunpower and Shell Solar are among the main manufacturers of this type of cells.
- Poly or multi crystalline made from cast ingots - large crucibles of molten silicon carefully cooled and solidified. These cells are cheaper than single crystal cells but are less efficient (typically ~15–16%). However, they can easily be formed into square shapes that cover a greater fraction of a panel than monocrystalline cells, and this compensates for their lower efficiencies. See GT Solar HEM Furnace, BP Solar, Sharp Solar and Kyocera Solar.
- Ribbon silicon, formed by drawing flat thin films from molten silicon and having a multicrystalline structure. These cells have lower efficiencies (~13.5–15%), but there is an additional cost saving since there is very little silicon waste, as this approach does not require sawing from ingots. See Evergreen Solar, and RWE Schott Solar for examples of commercialized technologies for growing vertical ribbon without assistance of a substrate. Examples of horizontally grown ribbon with the assistance of a substrate include GE Solar's SiliconFilm™ technology (formerly AstroPower) and Ribbon Growth on Substrate (RGS).
These technologies are wafer-based manufacturing. In other words, in each of the above approaches, self-supporting wafers between 180 to 240 micrometers thick are processed into solar cells and then soldered together to form a module.
Thin film approaches are module-based. The entire module substrate is coated with the desired layers and a laser scribe is then used to delineate individual cells. Two main thin film approaches are amorphous silicon and CIS:
- Amorphous silicon films are fabricated using chemical vapor deposition techniques, typically plasma enhanced (PE-CVD). These cells have low efficiencies of around 8%, however they are much less costly to produce. Amorphous silicon has a higher bandgap (1.7 eV) than crystalline silicon (c-Si) (1.1 eV), which means it is more efficient to absorb the visible part of the solar spectrum, but it fails to collect an important part of the spectrum : the infrared. As nanocrystalline Si has about the same bandgap as c-Si, the two material can be combined by depositing two diodes on top of each other creating a layered cell called a tandem cell. The top cell in a-Si absorbs the visible light and leaves the infrared part of the spectrum for the bottom cell in nanocrystalline Si, as pioneered by the Sanyo HIT cell. A patented silicon thin film technology being developed by XsunX, Inc, for building integrated photovoltaics (BIPV) in the form of semi-transparent solar cells which can be applied as window glazing. These cells function as window tinting while generating electricity.
- CIS stands for general chalcogenide films of Cu(InxGa1-x)(SexS1-x)2. While these films can achieve 11% efficiency, their costs are still too high.
- Dye-sensitized solar cells (sometimes called photoelectrochemical cells or Graetzel cells) were first announced in Nature in October 1991. These cells were initially conceptualized to mimick the process of photosynthesis. The dye-sensitized cell depends on a layer of nanoparticulate titanium dioxide, using a metalorganic dye (Ru-centered) as a light-absorbing material. The photogenerated electrons are passed on to the n-type TiO2 and the holes are passed to an electrolyte on the other side of the dye. The circuit is completed by a redox couple in the electrolyte, which can be liquid or solid. This type of cell allows a more flexible use of materials, and typically are manufactured by screen printing, with cost advantages over the more expensive manufacturing techniques and equipments used for traditional and thin film solar cells, and significantly less embodied energy. However, the dyes in these cells also suffer from degradation under heat and UV light, and the cell casing is difficult to seal due to the solvents used in assembly. In spite of the above, this is a popular emerging technology with some commercial impact forecasted within this decade.
The chart below illustrates the various commercial large-area module efficiencies and the best laboratory efficiencies obtained for various materials and technologies.
Interconnection and modules
Usually, solar cells are electrically connected, and combined into "modules", or solar panels. Solar panels have a sheet of glass on the front, and a resin encapsulation behind to keep the semiconductor wafers safe from the elements (rain, hail, etc). Solar cells are usually connected in series in modules, so that their voltages add.
Theory
Background
In order to understand how a solar cell works, a little background theory in semiconductor physics is required. For simplicity, the description here will be limited to describing the workings of single crystalline silicon solar cells.
Silicon is a group 14 (formerly, group IV) atom. This means that each Si atom has 4 valence electrons in its outer shell. Silicon atoms can covalently bond to other silicon atoms to form a solid. There are two basic types of solid silicon, amorphous (having no long range order) and crystalline (where the atoms are arranged in an ordered three dimensional array). There are various other terms for the crystalline structure of silicon; poly-crystalline, micro-crystalline, nano-crystalline etc, and these refer to the size of the crystal "grains" which make up the solid. Solar cells can be, and are made from each of these types of silicon, the most common being poly-crystalline.
Silicon is a semiconductor. This means that in solid silicon, there are certain bands of energies which the electrons are allowed to have, and other energies between these bands which are forbidden. These forbidden energies are called the "band gap". The allowed and forbidden bands of energy are explained by the theory of quantum mechanics.
At room temperature, pure silicon is a poor electrical conductor. In quantum mechanics, this is explained by the fact that the Fermi level lies in the forbidden band-gap. To make silicon a better conductor, it is "doped" with very small amounts of atoms from either group 13 (III) or group 15 (V) of the periodic table. These "dopant" atoms take the place of the silicon atoms in the crystal lattice, and bond with their neighbouring Si atoms in almost the same way as other Si atoms do. However, because group 13 atoms have only 3 valence electrons, and group 15 atoms have 5 valence electrons, there is either one too few, or one too many electrons to satisfy the four covalent bonds around each atom. Since these extra electrons, or lack of electrons (known as "holes") are not involved in the covalent bonds of the crystal lattice, they are free to move around within the solid. Silicon which is doped with group 13 atoms (aluminium, gallium) is known as p-type silicon because the majority charge carriers (holes) carry a positive charge, whilst silicon doped with group 15 atoms (phosphorus, arsenic) is known as n-type silicon because the majority charge carriers (electrons) are negative. It should be noted that both n-type and p-type silicon are electrically neutral, i.e. they have the same numbers of positive and negative charges, it is just that in n-type silicon, some of the negative charges are free to move around, while the converse is true for p-type silicon.
Light generation of carriers
When a photon of light hits a piece of silicon, one of three things can happen. The first is that the photon can pass straight through the silicon. This (generally) happens when the energy of the photon is lower than the bandgap energy of the silicon semiconductor. The second thing that can happen is that the photon is reflected off the surface. The third thing is that it can be absorbed by the silicon. This (generally) happens if the photon energy is greater than the bandgap energy of silicon. When a photon is absorbed, its energy is given to an electron in the crystal lattice. Usually this electron is in the valence band, and is tightly bound in covalent bonds between neighboring atoms, and hence unable to move far. The energy given to it by the photon "excites" it into the conduction band, where it is free to move around within the semiconductor. The covalent bond that the electron was previously a part of now has one less electron - this is known as a hole. The presence of a missing covalent bond allows the bonded electrons of neighboring atoms to move into the "hole," leaving another hole behind, and in this way a hole can move through the lattice. Thus, it can be said that photons absorbed in the semiconductor create mobile electron-hole pairs.
A photon need only have greater energy than that of the band gap in order to excite an electron from the valence band into the conduction band. However, the solar frequency spectrum approximates a black body spectrum at ~6000 K, and as such, much of the solar radiation reaching the Earth is composed of photons with energies greater than the band gap of silicon. These higher energy photons will be absorbed by the solar cell, but the difference in energy between these photons and the silicon band gap is converted into heat (via lattice vibrations - called phonons) rather than into usable electrical energy.
The p-n junction
The most commonly known solar cell is a large-area semiconductor p-n junction. To understand the workings of a p-n junction it is convenient to imagine what happens when a piece of n-type silicon is brought into contact with a piece of p-type silicon. In practice, however, the p-n junctions of solar cells are not made in this way, but rather, usually, by diffusing an n-type dopant into one side of a p-type wafer.
If we imagine what happens when a piece of p-type silicon is placed in intimate contact with a piece of n-type silicon, then what occurs is a diffusion of electrons from the region of high electron concentration - the n-type side of the junction, into the region of low electron concentration - p-type side of the junction. When the electrons diffuse across the p-n junction, they recombine with holes on the p-type side. This diffusion of carriers does not happen indefinitely however, because of the electric field which is created by the imbalance of charge immediately either side of the junction which this diffusion creates. Electrons from donor atoms on the n-type side of the junction are crossing into the p-type side, leaving behind the (extra) positively charged nuclei of the group 15 donor atoms, leaving an excess of positive charge on the n-type side of the junction. At the same time, these electrons are filling in holes on the p-type side of the junction, becoming involved in covalent bonds around the group 13 acceptor atoms, making an excess of negative charge on the p-type side of the junction. This imbalance of charge across the p-n junction sets up an electric field which opposes further diffusion of charge carriers across the junction.
This region where electrons have diffused across the junction is called the depletion region because it no longer contains any mobile charge carriers. It is also known as the "space charge region".
The electric field which is set up across the p-n junction creates a diode, allowing current to flow in only one direction across the junction. Electrons may pass from the n-type side into the p-type side, and holes may pass from the p-type side to the n-type side. But since the sign of the charge on electrons and holes is opposite, conventional current may only flow in one direction.
Separation of carriers by the p-n junction
Once the electron-hole pair has been created by the absorption of a photon, the electron and hole are both free to move off independently within the silicon lattice. If they are created within a minority carrier diffusion length of the junction, then, depending on which side of the junction the electron-hole pair is created, the electric field at the junction will either sweep the electron to the n-type side, or the hole to the p-type side.
Connection to an external load
Ohmic metal-semiconductor contacts are made to both the n-type and p-type sides of the solar cell, and the electrodes connected to an external load. Electrons that are created on the n-type side, or have been "collected" by the junction and swept onto the n-type side, may travel through the wire, power the load, and continue through the wire until they reach the p-type semiconductor-metal contact. Here, they recombine with a hole that was either created as an electron-hole pair on the p-type side of the solar cell, or swept across the junction from the n-type side after being created there.
Equivalent circuit of a solar cell
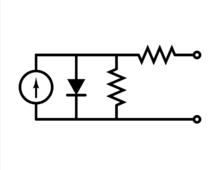
To understand the electronic behaviour of a solar cell, it is useful to create a model which is electrically equivalent, and is based on discrete electrical components whose behaviour is well known. An ideal solar cell may be modelled by a current source in parallel with a diode. In practice no solar cell is ideal, so a shunt resistance and a series resistance component are added to the model. The result is the "equivalent circuit of a solar cell" shown on the left. Also shown on the right, is the schematic representation of a solar cell for use in circuit diagrams.
Manufacture and devices
Because solar cells are semiconductor devices, they share many of the same processing and manufacturing techniques as other semiconductor devices such as computer and memory chips. However, the stringent requirements for cleanliness and quality control of semiconductor fabrication are a little more relaxed for solar cells.
Most large-scale commercial solar cell factories today make screen printed poly-crystalline silicon solar cells. Single crystalline wafers which are used in the semiconductor industry can be made in to excellent high efficiency solar cells, but they are generally considered to be too expensive for large-scale mass production.
Poly-crystalline silicon wafers are made by wire-sawing block-cast silicon ingots into very thin (250 to 350 micrometer) slices or wafers. The wafers are usually lightly p-type doped.
To make a solar cell from the wafer, an n-type diffusion is performed on the front side of the wafer, forming a p-n junction a few hundred nanometers below the surface.
Antireflection coatings, which increase the amount of light coupled into the solar cell, are typically applied next. Over the past decade, silicon nitride has gradually replaced titanium dioxide as the antireflection coating of choice because of its excellent surface passivation qualities (i.e., it prevents carrier recombination at the surface of the solar cell). It is typically applied in a layer several hundred nanometers thick using plasma-enhanced chemical vapor deposition (PECVD).
The wafer is then metallized, whereby a full area metal contact is made on the back surface, and a grid-like metal contact made up of fine "fingers" and larger "busbars" is screen-printed onto the front surface using a silver paste. The rear contact is also formed by screen-printing a metal paste, typically aluminum. Usually this contact covers the entire rear side of the cell, though in some cell designs it is printed in a grid pattern. The metal electrodes will then require some kind of heat treatment or "sintering" to make Ohmic contact with the silicon.
After the metal contacts are made, the solar cells are interconnected in series (and/or parallel) by flat wires or metal ribbons, and assembled into modules or "solar panels". Solar panels have a sheet of tempered glass on the front, and a polymer encapsulation on the back. Tempered glass cannot be used with amorphous silicon cells because of the high temperatures during the deposition process.
Some solar cells have textured front surfaces that, like antireflection coatings, serve to increase the amount of light coupled into the cell. Such surfaces can usually only be formed on single-crystal silicon, though in recent years methods of forming them on multicrystalline silicon have been developed.
Energy conversion efficiency
Typical module efficiencies for commercially available screen printed multicrystalline solar cells are around 12%. However, efficiencies vary from 6% for amorphous silicon-based solar cells to 30% or higher with multiple-junction research lab cells. A solar module's energy conversion efficiency, (or just efficiency) is the ratio of the maximum output electrical power divided by the input light power under "standard" test conditions. The "standard" solar radiation (known as the "air mass 1.5 spectrum") has a power density of 1000 watts per square meter. Thus, 1 m² of typical multicrystalline solar panels in full sunlight at solar noon at the equator during either the March or September equinox will produce approximately 120 watts of peak power. A more technical description of efficiency is the maximum power, made up of the fill factor (the percentage of the panel face filled up by solar cells vs the space between cells) x the open circuit voltage x the short circuit current, divided by the input power.
Note : A typical 4 square centimeter solar cell produces electrical energy of the order of 0.4 to 0.5 volts at 6 milliamperes.
Applications and implementations
See the article solar panel for information about applications and implementations of solar cells and panels.
Cost analysis
Based on manufacturer-reported power-output ratings, the mean US retail module price is $5.32/Wp with a 10th-percentile price of about $4.50/Wp (see SolarBuzz). Additional installation costs for a residential rooftop retrofit in California (CA) is around $3.50/Wp or more. So on the low side, installed system costs are about $7.00/Wp in CA, and probably higher in places with less experience. Federal, state, utility, and other subsidies combined can pay about half this cost depending on location. See link DSIRE (the Database of State Incentives for Renewable Energy) to determine applicable incentives for a given area.
Under net metering, one offsets regular retail utility rate which for CA PG&E residential customers is 12 cents/kWh, for tier 1 rates including tax [1]. Average customers are exposed to tier 3 rates of 22 cents/kWh. With a time of use meter, customers can offset some peak summer tier 3 rates of 40 cents. Commercial and agricultural customers are exposed to higher rates.
Knowing installed system costs, amount of sunshine, and the utility rates, one can figure out the years till payback with or without financing costs. Assuming no financing costs and a $6/Wp installed system cost (lower than current $7), one can take sunshine and utility rate information from around the globe and come up with a payback graph such as shown below. The addition of subsidies brings down the years to payback proportionately. For example, if the years to payback were 24 years at $6/Wp, and subsidies brought that down to $3/Wp, the years to payback would be 12.
When calculating the expected return on investment for Solar PV versus other investments, one might also take into account predicted increases in nominal retail electric rates. Additionally, locking in fixed rates via Solar PV provides a hedge against volatile utility rates, and this hedge has a separate monetary value. Since his home-generated electrical service might be considered a boutique electrical service, the dedicated PV enthusiast might also want to calculate his payback by comparing against boutique electric rates instead of against simply the lowest rates available to the public. SMUD suggests that boutique electricity is worth at least 10% more than non-green electricity [2].
Current research
There are currently many research groups active in the field of photovoltaics at universities and research institutions around the world.
Much of the research is focused on making solar cells cheaper and/or more efficient, so that they can more effectively compete with other energy sources, including fossil energy. One way of doing this is to develop cheaper methods of obtaining silicon that is sufficiently pure. Silicon is a very common element, but is normally bound in silica sand.
The current industrial production of silicon is via the reaction between carbon (charcoal) and silica at a temperature around 1700 degrees Celsius. In this process, known as carbothermic reduction, each tonne of silicon (metallurgical grade, about 98% in purity) is produced with the emission of about one and half tonnes of carbon dioxide.
It is recently reported that solid silica can be directed converted (reduced) to pure silicon by electrolysis in a molten salt bath at a fairly mild temperature (800 to 900 degrees Celsius). [refs. T. Nohira et al, ‘Pinpoint and bulk electrochemical reduction of insulating silicon dioxide to silicon’, Nat. Mater., 2 (2003) 397. X. B. Jin et al, Electrochemical preparation of silicon and its alloys from solid oxides in molten calcium chloride’, Angew. Chem. Int. Ed., 43 (2004) 733.] While this new process is in principle the same as the FFC Cambridge Process which was first discovered in late 1996, the interesting laboratory finding is that such produced electrolytic silicon is in the form of porous silicon which turns readily into a fine powder (particle size: a few micrometers), and may therefore offer new opportunities for development of solar cell technologies.
Another approach is to significantly reduce the amount of raw material used in the manufacture of solar cells. The various thin-film technologies currently being developed make use of this approach to reducing the cost of electricity from solar cells.
One approach to reduce the amount of silicon used and thus cost, as done by Australian National University in production of their "Sliver" cells, is to micromachine wafers into very thin, practically transparent layers, that could be used as transparent architechtural coverings. Using this technique, two silicon wafers are enough to build a 140 W panel, compared to about 60 wafers needed for conventional modules of same power output.
The invention of conductive polymers, (for which Alan Heeger was awarded a Nobel prize) may lead to the development of much cheaper cells that are based on inexpensive plastics, rather than semiconductor grade silicon. However, all organic solar cells made to date suffer from degradation upon exposure to UV light, and hence have lifetimes which are far too short to be viable.
Because photovoltaic panels convert a small fraction of the received light energy to electricity, there has been continued interest in laminating photovoltaic cells onto solar thermal panels to make PVT panels. Research in this area has found many difficulties and not much success (see References), for four reasons:
- Photovoltaic panels are usually designed to reflect light not used, rather than absorb it, and the difference requires quite a bit of development.
- Solar thermal panels for domestic hot water or space heating are usually glazed, and the glass tends to reflect some of the light that the PV cell might absorb.
- Because the panels convert less light to heat, they require more area for the same heat and suffer larger radiative and convective losses, all of which reduces the overall cost effectiveness of the solar thermal system.
- Because the manufacturing process is less developed, costs are higher, which is crippling in the extremely cost-sensitive solar market.
Thin-film solar cells
The next step in reducing the cost of solar cells and panels seems certain to come from thin-film technology. Thin-film solar cells use less than 1% of the raw material (silicon) compared to wafer based solar cells, leading to a significant price drop per kWh. There are many research groups around the world actively researching different thin-film approaches and/or materials.
Thin Film solar cells are mainly deposited by PECVD from silane gas and hydrogen. This process produces a material without crystalline orientation : amorphous silicon. Depending on the deposition's parameters both protocrystalline silicon, which has been shown to exhibit the most stability, and nanocrystalline silicon can also be obtained. These types of silicon present dandling and twisted bonds, which results in the aparition of deep defects (energy levels in the bandgap) as well as in the deformation of the valence and conduction bands (band tails). This contributes to reduce the efficiency of Thin-Film solar cells by reducing the number of collected electron-hole pair by incident photon.
Amorphous silicon (a-Si) has a higher bandgap (1.7 eV) than crystalline silicon (c-Si) (1.1 eV), which means it is more efficient to absorb the visible part of the solar spectrum, but it fails to collect an important part of the spectrum : the infrared. As nano crystalline Si has about the same bandgap as c-Si, the two material can be combined by depositing two diodes on top of each other : the tandem cell. The top cell in a-Si absorbs the visible light and leaves the infrared part of the spectrum for the bottom cell in nanocrystalline Si.
One particularly promising technology is crystalline silicon thin-films on glass substrates. This technology makes use of the advantages of crystalline silicon as a solar cell material, with the cost savings of using a thin-film approach.
Another interesting aspect of thin-film solar cells is the possibility to deposit the cells on all kind of materials, including flexible substrates (PET for example), which opens a new dimension for new applications.
Emerging materials
For special applications, such as Deep Space 1, high-efficiency cells can be made from gallium arsenide by molecular beam epitaxy. Such cells have many diodes in series, each with a different band gap energy so that it absorbs its share of the electromagnetic spectrum with very high efficiency. Triple junction solar cell have (as the name suggest) 3 diodes layered on top of each other, each absorbing a different spectrum of light, efficiency as high as 35.2% have been achieved. The multiple junction solar cells may be very efficient, but are prohibitively expensive to make. Cost-effective use of these cells could be achieved with concentrating optics so that less of the array consists of actual semiconductor devices.
Experimental non-silicon solar panels can be made of quantum heterostructures, eg. carbon nanotubes or quantum dots, embedded in a special plastic. These have only one-tenth the efficiency of silicon panels but could be manufactured in ordinary factories, not clean rooms which should lower the cost. While conventional solar cells only generate electricity from the visible light spectrum, experimental cells have been made that use the infrared spectrum. By varying the size of the quantum dots, the cells can be tuned to absorb different wavelengths. If panels that absorb both visible and infrared spectrums are able to be manufactured, the panels may be able to achieve up to 30 percent efficiency. (McDonald, et al., 2005)
Some of the most efficient solar cell materials are cadmium telluride (CdTe) and copper indium gallium selenide (CIGS). Unlike the basic silicon solar cell, which can be modelled as a simple p-n junction (see under semiconductor), these cells are best described by a more complex heterojunction model. The best efficiency of a bare thin-film solar cell as of December 2005 was 19.5% (CIGS). Higher efficiencies (around 30%) can be obtained by using optics to concentrate the incident light.
Polymer solar cells, also called organic solar cells, are built from ultra thin layers (typically 100 nm) of organic semiconductors such as polyphenylene vinylene and fullerene. The p/n junction model is only a crude description of the functioning of such cells, as electron hopping and other processes also play a crucial role. They are potentially cheaper to manufacture than silicon or inorganic cells, but efficiencies achieved to date are low and cells are highly sensitive to air and moisture, making commercial applications difficult. In the reverse mode, the technology has however already successfully been commercialised in organic LEDs and organic displays, also called polymer displays.
Graetzel cells (sometimes called photoelectrochemical cells or dye-sensitized solar cells) were first announced in Nature in October 1991. The cell depends on a layer of nanoparticulate titanium dioxide, sensitised by a dye. In contrast to the classical solar cell the dye absorbs the radiation, mimicking the process of photosynthesis. The circuit is completed by a redox couple in the electrolyte, which can be liquid or solid. As a result, this type of cell allows a more flexible use of materials, and typically are manufactured by screen printing, with cost advantages over the more expensive manufacturing techniques and equipments used for traditional and thin film solar cells, and significantly less embodied energy. This is an emerging technology with commercial impact forecast within this decade.
Solar cells and energy payback
There is a common but mistaken notion that solar cells never produce more energy than it takes to make them. While the expected working lifetime is around 40 years, the energy payback time of a solar panel is anywhere from 1 to 30 years (usually under five) depending on the type and where it is used (see net energy gain). This means solar cells are net energy producers and can "reproduce" themselves (from 6 to more than 30 times) over their lifetime. For details see Net Energy Analysis For Sustainable Energy Production From Silicon Based Solar Cells.
See also
- Autonomous building
- Future energy development
- Green technology
- Photodiode
- Photovore
- Renewable energy
- Solar power
- Solar panel
- Timeline of solar energy
References
- McDonald SA, Konstantatos G, Zhang S, Cyr PW, Klem EJ, Levina L, Sargent EH (2005). "Solution-processed PbS quantum dot infrared photodetectors and photovoltaics". Nature Materials. 4 (2): 138–42. PMID 15640806.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- PVNET European Roadmap for PV R&D Ed Arnulf Jager-Waldan Office for Publications of the European Union 2004
External links
- Use of solar cells in Kenya and Uganda, in Africa
- Pennicott, Katie, "Solar cell edges towards endless energy". 7 December 2001. PhysicsWeb.
- Dye Sensitized Solar Cells (DYSC) based on Nanocrystalline Oxide Semiconductor Films
- News searching: Solar Cell, Photovoltaic
- Historical: Photovoltaic Solar Energy Conversion: An Update
- Wladek Walukiewicz, Materials Sciences Division, Berkeley Lab.: Full Solar Spectrum Photovoltaic Materials Identified. Quote: "... Maximum, theoretically predicted efficiencies increase to 50%, 56%, and 72% for stacks of 2, 3, and 36 junctions with appropriately optimized energy gaps, respectively...."
- CNET: SunPower Announces World's Most Efficient, Low-Cost Silicon Solar Cell (12 May 2003) Quote: "...The National Renewable Energy Laboratory (NREL) has verified 20.4 percent conversion efficiency for the A-300...."
- SunPower A-300 (pdf), SunPower
- Scientists Create New Solar Cell (29 March 2002) Quote: "...semiconducting plastic material known as P3HT... 1.7 percent for sunlight..."
- 'Denim' solar panels to clothe future buildings (15 February 2003) Quote: "... Unlike conventional solar cells, the new, cheap material has no rigid silicon base..."
- Residential Solar Power Systems - Photo Gallery
- Examples of Photovoltaic Systems
- howstuffworks.com: How Solar Cells Work
- [http://www.azonano.com/news.asp?newsID=548 azonano.com: Carbon Nanotube Structures Could Provide More Efficient Solar Power(28 February 2005)
- Solar energy timeline
- PV-Thermal collector development -- an overview of the lessons learnt] Zondag et al, 2004
- http://news.nationalgeographic.com/news/2005/01/0114_050114_solarplastic.html>Spray-On Solar-Power Cells Are True Breakthrough
Yield data
- http://www.tectosol.staticip.de/index_en.htm electricity yield of a solar power system
- http://www.sunny-portal.de Yield Portal for solar power system users
Theory
- National Renewable Energy Laboratory (NREL): Photovoltaics for Buildings: PV Technology for the Home Factsheets
- 1993, National Renewable Energy Laboratory (NREL): Photovoltaics: Unlimited Electrical Energy From the Sun BrokenLink
- Electrical models of solar cells
Dye solar Cells
- [3] commercialising Dye Solar Cell technology
Cost Benefit
Do-it-yourself
- PEC (Photo Electro Chromic)
- How to Build Your Own Solar Cell
- DIY (Do It Yourself): Nanocrystalline Dye-Sensitized Solar Cell Kit Quote: "... sunlight-to-electrical energy conversion efficiency is between 1 and 0.5 %..."
- Cuprous oxide solar cells
- Make a solar cell in your kitchen, A flat panel solar battery
- From: How to Build a Solar Cell That Really Works by Walt Noon

