Barium peroxide

| |
| |
| Names | |
|---|---|
| IUPAC name
barium peroxide
| |
| Other names
Barium binoxide,
Barium dioxide, Barium superoxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.754 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaO2 | |
| Molar mass | 169.33 g/mol |
| Appearance | Grey-white crystalline solid |
| Density | 5.68 g/cm3 |
| Melting point | 450 °C (723 K) |
| Boiling point | 800 °C (1073 K) (decomp. to BaO & O2) |
| insoluble [1] | |
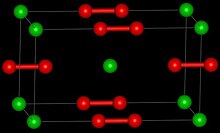
| Structure | |
| Tetragonal [2] | |
| D174h, I4/mmm, tI6 | |
| 6 | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium peroxide is the chemical compound with the formula BaO2. This grey-white solid is one of the most common inorganic peroxides. Barium peroxide is an oxidizing agent, which is used for bleaching. It is used in fireworks as an oxidizer,[3] which also gives a vivid green colour, as do all barium compounds.
Barium peroxide is a peroxide, containing O22− subunits wherein the oxygen atoms bond to each other as well as to the barium. The solid adopts the same structure as calcium carbide, CaC2.
Barium peroxide arises by the reversible absorption of O2 by barium oxide. The oxygen is released above 500°C.
- 2 BaO + O2 ⇌ 2 BaO2
This reaction is the basis for the now-obsolete Brin process for separating oxygen from the atmosphere. Other oxides, e.g. Na2O, behave similarly.[4]
Hydrogen peroxide can also be prepared via the related reaction:
- BaO2 + H2SO4 → H2O2 + BaSO4
The insoluble barium sulfate is filtered from the mixture.
Footnotes
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ Massalimov, I. A.; Kireeva, M. S.; Sangalov, Yu. A. (2002). Inorganic Materials. 38 (4): 363. doi:10.1023/A:1015105922260.
{{cite journal}}: Missing or empty|title=(help) - ^ "Data Sheet". Data Sheet. Hummel Croton Inc. Retrieved 2007-02-01.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
See also
External links

