Keratoconus
| Keratoconus | |
|---|---|
| Specialty | Ophthalmology, optometry |
Keratoconus (from Greek: kerato- horn, cornea; and Latin: conus cone), is a degenerative non-inflammatory disorder of the eye in which structural changes within the cornea cause it to thin and change to a more conical shape than its normal even curve. Keratoconus can cause substantial distortion of the vision, with multiple images, streaking and sensitivity to light all often reported by the patient. Though frequently thought of as an rare condition, keratoconus is the most common distrophy of the cornea, affecting around one person in a thousand, and seems to occur equally in all ethnic groups worldwide. It is typically diagnosed in the patient's adolescent years and attains its most severe state in the twenties and thirties.
Keratoconus is a little-understood disease with an uncertain cause, and the course of its progression following diagnosis is unpredictable. The associated deterioration in vision, if in both eyes, can affect the person's ability, for example, to drive a car legally. It does not however lead to blindness, and in most cases, corrective lenses are effective enough to allow the patient to continue to drive and likewise function normally. Further progression of the disease may lead to a need for surgery. There is no known cure for keratoconus, nor can its natural progression be significantly arrested, but it can be successfully managed often with little or no impairment to quality of life.
History
In a 1748 doctoral dissertation, the German oculist Burchard Mauchart provided an early description of a case of keratoconus, which he referred to as staphyloma diaphanum. His treatise, and those of others, do not however seem to have received much attention in the contemporary literature, and it would not be until 1854 that keratoconus was properly described and distinguished from other deformities of the cornea by John Nottingham.[1] Nottingham's text reported many cases of 'conical cornea' that had come to his attention, and described several of the classic features of the disease, including polyopia, the weakness of the cornea, and the difficulty in matching spectacle lenses to the patient's vision. Clinical knowledge of keratoconus took another step forward when in 1859 William Bowman applied Helmholtz's recent invention of the ophthalmoscope to its diagnosis, describing how to angle the instrument's mirror so to throw light on the cornea from different directions; the side of the cone opposite the light appearing darker than elsewhere.[2] The pioneering Swiss ophthalmologist Johann Horner wrote a thesis titled On the treatment of keratoconus[3] in 1869, by which time the disorder had acquired its current name. The treatment attempted to physically reshape the cornea by chemical cauterization with a silver nitrate solution, and application of a miosis-causing agent to the eye with a pressure dressing. When in March 1888 the French physician Eugene Kalt presented a paper describing a glass scleral shell he had designed which improved vision by compressing the cornea into a more regular shape,[4] the treatment of keratoconus became one of the first practical applications of the then newly-invented contact lens. Since the start of the twentieth century, research on keratoconus has extended the knowledge base of the disease's pathology and causes; and refined and extended the range of clinical options.
Features
Symptoms

Patients normally present initially with minor blurring of vision and seeking corrective lenses for reading or driving. As the disease continues, vision worsens and symptoms become more pronounced. The exact nature of the vision distortion introduced by keratoconus is most clearly seen with a high contrast field such as a point of light on a dark background – instead of seeing one point the patient may see over 100 images spread out in a complex and random pattern. The pattern does not change from day to day, but over the seasons it often takes on new forms. The effect can worsen in low light conditions as the dark-adapted pupil dilates to expose more of the irregular surface of the cornea.
The visual distortion experienced by the patient comes from two sources, one being the irregular deformation of the surface of the cornea; the other being scarring that occurs on its exposed highpoints. These factors act to form regions on the cornea that map an image to different locations on the retina, hence creating the perception of multiple images known as monocular polyopia. Scarring appears to be an aspect of the corneal degradation; however, a recent, large, multi-center study suggests that abrasion by contact lenses may increase the likelihood of this finding by a factor of over two.[5]
More severe symptoms
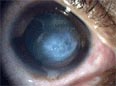
In advanced cases, bulging of the cornea can result in a localized rupture of Descemet's membrane, an inner layer of the cornea. Aqueous humor from the eye's anterior chamber seeps into the cornea before Descemet's membrane reseals. The patient experiences pain and a sudden severe clouding of vision, with the cornea taking on a translucent milky-white appearance known as a corneal hydrops.[6] Although disconcerting to the patient, the effect is normally temporary and after a period of six to eight weeks the cornea usually returns to its former transparency. The recovery can be aided non-surgically by bandaging with an osmotic saline solution. Although a hydrops usually causes increased scarring of the cornea, occasionally it will benefit a patient by creating a flatter cone, aiding the fitting of contact lenses.[6] Very occasionally, in extreme cases, the cornea thins to the point that a partial rupture occurs, resulting in a small, bead-like swelling on the cornea that has been filled with fluid. When this occurs, a transplant can become urgently necessary to avoid complete rupture and resulting loss of the eye.[6]
Diagnosis
An ophthamologist will normally subject the patient to an initial vision test with a standard Snellen chart of progressively smaller letters, and inquire into the patient's general medical history with an interest in any prior disease or injury which might affect vision. The eye examination may proceed to measurement of the localised curvature of the cornea with a manual keratometer,[7] with detection of irregular astigmatism suggesting a possibility of keratoconus. Severe cases can exceed the instrument's measuring ability. A further indication can be provided by retinoscopy, in which a light beam is focused on the patient's retina and the reflection, or reflex, observed as the examiner tilts the light source back and forth. Keratoconus is amongst the ophthalmic conditions that exhibit a scissor reflex action of two bands moving toward and away from each other like the blades of a pair of scissors.[8]
If keratoconus is suspected, the ophthamologist will search for other characteristic findings of the disease by means of slit lamp examination of the cornea. An advanced case is usually readily apparent to the examiner, and can provide for an unambiguous diagnosis prior to more specialised testing. Under close examination, a ring of yellow-brown to olive-green pigmentation known as a Fleischer ring can be observed in around half of keratoconic eyes,[9] and is caused by deposition of the iron oxide hemosiderin within the corneal epithelium. The Fleischer ring is subtle, and may not be readily detectable in all cases, but becomes more evident when viewed under a cobalt blue filter. Similarly, around 50% of subjects exhibit Vogt's striae, fine stress lines within the cornea caused by stretching and thinning of the protruded cone.[9] The striae temporarily disappear when slight pressure is applied to the eyeball. A highly pronounced cone can create a V-shaped indentation in the lower eyelid when the patient's gaze is directed downwards, known as Munson's sign. Other clinical signs of keratoconus will normally have presented themselves long before Munson's sign becomes apparent,[10] and so this finding, though a classic sign of the disease, tends not to be of primary diagnostic importance.
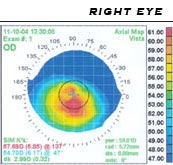
A handheld keratoscope, sometimes known as Placido's disk, can provide the examiner with a simple non-invasive visualization of the surface of the cornea by projecting a series of concentric rings of light onto the cornea. A more definitive diagnosis of keratoconus can be obtained using corneal topography, in which an instrument projects the illuminated pattern onto the cornea and and infers its topology by computer analysis. The topographical map indicates any distortions or scarring present in the cornea, with keratoconus revealed by a characteristic steepening of curvature which is usually inferior to (below) the centreline of the eye. The technique can record a snapshot of the degree and extent of the deformation as a benchmark for assessing its rate of progression. It is of particular value in providing an detection of the disorder in its early stages when other signs have not yet presented.[11]
Once the presence of keratoconus has been established, its degree may be classified by a number of means helpful to the examiner:[12][13]
- The steepness of greatest curvature from mild (< 45 D), advanced (up to 52 D) or severe (> 52 D);
- The morphology of the cone: nipple (small: 5 mm and near-central), oval (larger, below-center and often sagging), or globus (more than 75% of cornea affected);
- The corneal thickness from mild (> 506 μm) to advanced (< 446 μm).
Increasing use of corneal topography has led to a decline in the use of these terms by some practitioners.[13]
Epidemiology
The National Eye Institute reports that keratoconus is the most common corneal dystrophy in the United States, affecting approximately 1 in every 2,000 Americans, [14][15] but some reports place the figure as high as 1 in every 500.[16] The inconsistency may be due to variations in diagnostic criteria, with some cases of high astigmatism intepreted as those of keratoconus, and vice versa.[10] A long-term study found a mean incidence rate of 2.0 new cases per 100,000 population per year.[15] Males and females, and all ethnicities appear equally susceptible, though some recent studies have cast doubt upon this,[17] suggesting a higher prevalence amongst females; the literature however varying as to its extent. Keratoconus is normally bilateral[15], affecting both eyes, although the distortion is usually asymmetric and is rarely completely identical in both corneas. Unilateral cases tend to be uncommon, and may in fact be very rare if a very mild condition in the better eye is simply below the limit of clinical detection.[10] It is common for keratoconus to be diagnosed first in one eye and not until later in the other. As the condition then progresses in both eyes, the vision in the earlier eye will often persist to be poorer than that in its fellow.
Prognosis
Patients with keratoconus typically present initially with mild astigmatism, commonly at the onset of puberty, and are diagnosed as having the disease by the late teenage years or early 20s. In rare cases keratoconus can occur in children or not present until later adulthood. A diagnosis of the disease at an early age may indicate a greater risk of severity in later life.[18] Patients' vision will seem to fluctuate over a period of months, driving them to change lens prescriptions frequently but as the condition worsens, contact lenses become required in the majority of cases. The course of the disorder can be quite variable, with some patients remaining stable for years or indefinitely, while others progress rapidly or experience occasional exacerbations over a long and otherwise steady course. Most commonly, keratoconus progresses for a period of ten to twenty years[10] before the course of the disease generally ceases.
Pathophysiology and cause
Despite considerable research, the etiology of keratoconus remains somewhat of a mystery. According to the United States National Keratoconus Foundation,[19] it is likely that keratoconus can arise from a number of different factors: genetic, environmental or cellular, any of which may form the trigger for the onset of the disease. Once initiated, the disease normally develops by progressive dissolution of Bowman's layer, the membrane lying between the corneal epithelium and stroma. As the two come into contact, cellular and structural changes in the cornea adversely affect its integrity and lead to the bulging and scarring that are characteristic of the disorder. Within any individual keratoconic cornea, there may be found regions of degenerative thinning coexisting with regions undergoing wound healing.
A number of studies have indicated that keratoconic corneas show signs of increased activity by proteases, a class of enzymes that break some of the collagen cross-linkages in the corneal stroma, with a simultaneous reduced expression of protease inhibitors.[20] Other studies have suggested that reduced activity by the enzyme aldehyde dehydrogenase may be responsible for a build-up of free radicals and oxidising species in the cornea.[21] It seems likely that, whatever the pathogenetical process, the damage caused by activity within the cornea results in a reduction in its thickness, biomechanical strength and stiffness.
A genetic predisposition to keratoconus has been observed,[22] with the disease running in certain families,[23] and incidences reported of concordance in identical twins. The frequency of occurrence in close family members is not clearly defined and studies have obtained estimates ranging between 6% and 19%,[24] an incidence considerably higher than that in the general population. Putative gene locations were contrarily mapped to chromosomes 16q in one study and 20q in another[24] of isolated, largely homogenetic communities, though most genetic studies agree on a dominant autosomal model of inheritence. Keratoconus is also diagnosed more often in people with Down syndrome, though the reasons for this link have not yet been determined.[25] Keratoconus has been associated with atopic diseases, which include asthma, allergies, and eczema, and it is not uncommon for several or all of these diseases to affect one person. A number of studies suggest that vigorous eye rubbing may contribute to the progression of keratoconus, and that patients should be discouraged from the practice.[26][27][28][29][30]
Treatment
Contact lenses
In early stages of keratoconus, spectacles can suffice to correct for the mild astigmatism. As the condition progresses, spectacles may fail to provide the patient with a satisfactory degree of visual acuity, and most clinical practitioners will move to managing the condition with contact lenses.

In keratoconic patients, contact lenses improve vision by means of tear fluid filling the gap between the irregular corneal surface and the smooth regular inner surface of the lens, thereby creating the effect of a smoother cornea. Traditionally, lenses for keratoconus have been the "hard" or rigid gas-permeable contact lens variety, although recently somewhat effective "soft" or hydrophilic lenses have become available. Hybrid lenses have been recently developed which are hard in the centre and surrounded by a soft skirt around the edge. These soft or hybrid lenses are not effective for every patient.[31]
Many specialized types of gas permeable contact lenses have been developed for keratoconus, and affected people should seek out both doctors specialized in conditions of the cornea, and contact-lens fitters who have experience managing patients with keratoconus. The irregular cone presents a challenge and the fitter will endeavour to produce a lens with the optimal contact, stability and steepness. Some trial-and-error fitting may prove necessary.[10]
Some patients also find good vision correction and comfort with a "piggyback" lens combination, in which gas permeable rigid lenses are worn over soft lenses, both providing a degree of vision correction. One form of piggyback lens makes use of a soft lens with a countersunk central area to accept the rigid lens. Fitting a piggyback lens combination requires experience on the part of the lens fitter, and tolerance on the part of the keratoconic patient.
Scleral lenses are sometimes prescribed for cases of advanced or very irregular keratoconus; these lenses cover a greater proportion of the surface of the eye and hence can offer improved stability.[32] The larger size of the lenses may make them unappealing or uncomfortable to some, however their easier handling can find favor with patients with reduced dexterity, such as the elderly.
Surgical options
Corneal transplant

Between 10% to 25% of cases of keratoconus[19][33][34] will progress to a point where vision correction is no longer possible, thinning of the cornea becomes excessive, or scarring as a result of contact lens wear causes problems of its own, and a corneal transplantation or penetrating keratoplasty becomes required. Keratoconus is the most common grounds for conducting a penetrating keratoplasty, accounting for around a quarter of such procedures.[35] The corneal transplant surgeon uses a device called a trephine which acts like a cookie-cutter, cutting a lenticule, or button, of corneal tissue from the eye. The surgeon then sews the donor tissue to the existing eye tissue, usually using a combination of running and individual sutures. The cornea does not have a direct blood supply, and so donor tissue is not required to be blood type matched. Eye banks check the donor corneas for any disease or cellular irregularities.
The acute recovery period can take four to six weeks and full post-operative vision stabilization often takes a year or more but most transplants are very stable in the long term.[34] The National Keratoconus Foundation reports that penetrating keratoplasty has the most successful outcome of all transplant procedures, and when performed for keratoconus in an otherwise healthy eye, its success rate can be 95% or greater.[19] The sutures used usually dissolve over a period of three to five years but individual sutures can be removed during the healing process if they are causing irritation to the patient.
Cornea transplants for keratoconus are usually performed under sedation as outpatient surgery, and require careful follow-up with an eye surgeon for a number of years. Frequently, vision is greatly improved after the surgery, but even if the actual visual acuity does not improve, because the cornea is a more normal shape after the healing is completed, patients can more easily be fitted with corrective lenses. Complications of corneal transplants are mostly related to vascularization of the corneal tissue and rejection of the donor cornea. Vision loss is very rare, though difficult-to-correct vision is possible. When rejection is severe, repeat transplants are often attempted, and are frequently successful.[36] Keratoconus will not normally reoccur in the transplanted cornea; incidences of this have been observed, but are usually attributed to incomplete excision of the original cornea or inadequate screening of the donor tissue.[37] The long-term outlook for corneal transplants performed for keratoconus is usually favorable once the initial healing period is completed and a few years have elapsed without problems.
Epikeratophakia
Rarely, a non-penetrating keratoplasty known as an epikeratophakia (or epikeratoplasty) may be performed in cases of keratoconus. The corneal epithelium is removed and a lenticule of donor cornea grafted on top of it. The procedure requires a greater level of skill on the part of the surgeon, and is less frequently performed than a penetrating keratoplasty as the outcome is generally less favorable. It may however be seen as an option in a number of cases, particularly for young patients.[38]
Corneal ring segment inserts
A recent surgical alternative to corneal transplant is the insertion of intrastromal corneal ring segments. A small incision is made in the periphery of the cornea and two thin arcs of polymethyl methacrylate slid between the layers of the corneal stroma either side of the pupil, the incision then being closed. The segments push out against the curvature of the cornea, flattening the peak of the cone and returning it to a more natural shape. The procedure, carried out on an outpatient basis under local anaesthesia, offers the benefit of being reversible and even potentially exchangeable as it involves no removal of eye tissue.
The two principle types of intrastromal rings available are known by the trade names of Intacs and Ferrara rings. Intacs are flatter and less centrally placed than the prismatic Ferrara rings. Intacs were first approved by the FDA in the United States in 1999 for myopia; this was extended to the treatment of keratoconus in July 2004.[39] Ferrara rings await FDA approval for keratoconus. Further developments on the concept involve the injection of a transparent synthetic gel into a channel bored through the stroma. As the gel polymerises, it stiffens and takes on similar properties to the pre-formed rings.[40]
Clinical studies on the effectiveness of intrastromal rings on keratoconus are in their early stages, and results have so far been generally encouraging,[41][42] though they have yet to enter into wide acceptance with all refractive surgeons. In common with a penetrating keratoplasty, the requirement for some vision correction in the form of hydrophilic (soft) contact lenses or spectacles may remain subsequent to the operation. Potential complications of intrastromal rings include accidental penetration through to the anterior chamber when forming the channel, post-operative infection of the cornea, and migration or extrusion of the segments.[42] The rings offer a good chance of vision improvement even in otherwise hard to manage eyes, but it is not guaranteed and in a few cases may worsen.
Radial keratotomy
Radial keratotomy is a surgical procedure in which the surgeon makes non-penetrating incisions into the corneal epithelium and attempts to modify its topology by the controlled creation of corneal scar tissue. As an early surgical option for myopia, it was largely superseded by LASIK and similar procedures. LASIK itself is regarded as unsuitable for keratoconus due to the risk that removal of tissue would further damage an already weakened cornea.
Mainstream refractive surgery has generally regarded radial keratotomy as contraindicated for keratoconic patients,[43][44] though an Italian clinic has reported some success with a modified asymmetric radial keratotomy procedure,[45] in which the incisions are confined to a sector of the eye. The corneal thickness is first measured by means of a pachymeter and the surgeon makes cuts to a depth of between 70-80%. As with all forms of refractive surgery, the patient may experience photophobia and fluctuation of vision post-operation, but these are expected to stabilize in the medium term.[45]
Related disorders
There are several other non-inflammatory disorders, generally rarer than keratoconus, that also result in thinning of the cornea[10]:
- Keratoglobus
- Keratoglobus is a very rare condition with substantial thinning of the cornea, though most notably at the margins, resulting in a spherical, slightly enlarged eye. It may be genetically related to keratoconus.
- Pellucid marginal degeneration
- Pellucid marginal degeneration is the thinning of a narrow (1-2 mm) band of the cornea usually along the inferior margins, and causing substantial irregular astigmatism. It can normally be corrected by spectacles.
- Posterior keratoconus
- Despite the similarity in name, there are some significant differences between posterior keratoconus and 'normal' keratoconus. Posterior keratoconus is a rare abnormality, usually congenital, resulting in a thinning of the cornea from its inner surface. Posterior keratoconus is normally regular, non-progressive and usually affects only a single eye.
See also
References
- Arffa R (1997). Grayson's Diseases of the Cornea. Chap. 17. Mosby. ISBN 0815136544.
{{cite book}}: Cite has empty unknown parameters:|coauthors=and|month=(help) - Brown D. "Research Overview". National Keratoconus Foundation. Retrieved 2006-03-12.
- Burger D, Shovlin J, Zadnik K (2003). "Keratoconus: Diagnosis & Management". Pacific University College of Optometry. Retrieved 2006-03-12.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Caroline P, Andre M, Kinoshita B, and Choo, J. "Etiology, Diagnosis, and Management of Keratoconus: New Thoughts and New Understandings". Pacific University College of Optometry. Retrieved 2006-03-12.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Epstein A (2000). "Keratoconus and related disorders" (PDF). North Shore Contact Lens. Retrieved 2006-03-12.
- Feder R, Kshettry P (2005). "Chap 78: Non-inflammatory Ectactic Disorders". In Edited: Krachmer J (ed.). Cornea. Mosby. ISBN 0323023150.
{{cite book}}:|editor=has generic name (help); Cite has empty unknown parameters:|coauthors=and|month=(help) - Heverly V, Lowther G. "Keratoconus". School of Optometry, Indiana University. Retrieved 2006-03-12.
- Rabonitz Y (2004). "Ectatic Disorders of the Cornea". In Edited: Foster C; et al. (eds.). The Cornea (4th Ed. ed.). ISBN 0781742064.
{{cite book}}:|edition=has extra text (help);|editor=has generic name (help); Cite has empty unknown parameters:|coauthors=and|month=(help); Explicit use of et al. in:|editor=(help) - Sadick B (2000). "Signs and Signals of Keratoconus" (PDF). Retrieved 2006-03-12.
- Yanoff M, Duker J (2004). Ophthalmology (2nd Ed. ed.). Mosby. ISBN 0323016340.
{{cite book}}:|edition=has extra text (help); Cite has empty unknown parameters:|coauthors=and|month=(help) - Zadnik K, Barr J (1999). Diagnosis, Contact Lens Prescribing, and Care of the Keratoconus Patient. Butterworth Heinemann. ISBN 0750696761.
{{cite book}}: Cite has empty unknown parameters:|coauthors=and|month=(help)
Notes
- ^ Nottingham J. Practical observations on conical cornea: and on the short sight, and other defects of vision connected with it. London: J. Churchill, 1854. Canadian archives.
- ^ Bowman W, On conical cornea and its treatment by operation. Ophthalmic Hosp Rep and J R Lond Ophthalmic Hosp. 1859;9:157.
- ^ Horner JF, Zur Behandlung des Keratoconus. Klinische Monatsblätter für Augenheilkunde. 1869.
- ^ Kalt E, reported by Panas P, translated by Pearson R. Kalt, keratoconus and the contact lens. (1888). Bull Aced Med, 19, 400 Optom Vis Sci; (1989) 66, 643 PMID 2677884
- ^ Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, Zadnik K; CLEK Study Group. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2006 Jan;25(1):16-25. PMID 16331035
- ^ a b c Grewal S, Laibson PR, Cohen EJ, Rapuano CJ. Acute hydrops in the corneal ectasias: associated factors and outcomes. Trans Am Ophthalmol Soc. 1999;97:187-98; PMID 10703124
- ^ Nordan LT. Keratoconus: diagnosis and treatment. Int Ophthalmol Clin. 1997 Winter;37(1):51-63. PMID 9101345
- ^ Zadnik K (1997). The ocular examination : measurements and findings. Philadelphia: W.B. Saunders. ISBN 0721652093.
{{cite book}}: Cite has empty unknown parameters:|coauthors=and|month=(help) - ^ a b Edrington TB, Zadnik K, Barr JT. Keratoconus Optom Clin. 1995;4(3):65-73. PMID 7767020
- ^ a b c d e f Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984 Jan-Feb;28(4):293-322. PMID 6230745
- ^ Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. 1989 Aug 15;108(2):107-12. PMID 2757091
- ^ Caroline P, Andre M, Kinoshita B, and Choo, J. "Etiology, Diagnosis, and Management of Keratoconus: New Thoughts and New Understandings". Pacific University College of Optometry. Retrieved 2006-03-26.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ a b Gupta D. "Keratoconus: A clinical update" (PDF). Retrieved 2006-03-26.
- ^ US National Eye Institute, Facts About The Cornea and Corneal Disease Keratoconus. Accessed 12 Feb 2006.
- ^ a b c Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986 Mar 15;101(3):267-73. PMID 3513592
- ^ Weissman BA, Yeung KK. Keratoconus. eMedicine: Keratoconus. Accessed 12 Feb 2006.
- ^ Fink BA, Wagner H, Steger-May K, Rosenstiel C, Roediger T, McMahon TT, Gordon MO, Zadnik K. Differences in keratoconus as a function of gender. Am J Ophthalmol. 2005 Sep;140(3):459-68. PMID 16083843
- ^ Davis LJ. Keratoconus: Current understanding of diagnosis and management. Clin Eye Vis Care 9(I): 13-22, 1997.doi:10.1016/S0953-4431(96)00201-9
- ^ a b c Brown D. National Keratoconus Foundation: Research Overview. http://www.nkcf.org. Accessed 12 Feb 2006.
- ^ Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004 Jul;29(1):35-40. PMID 15370365
- ^ Gondhowiardjo TD et al.. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea. 1993 Mar;12(2):146-54. PMID 8500322
- ^ Edwards M, McGhee CN, Dean S. The genetics of keratoconus. Clin Experiment Ophthalmol. 2001 Dec;29(6):345-51. PMID 11778802
- ^ Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998 Dec;39(13):2537-46. PMID 9856763
- ^ a b Merin S (2005). Inherited Eye Disorders: Diagnosis and Management. Boca Raton: Taylor & Francis. ISBN 1574448390.
{{cite book}}: Cite has empty unknown parameters:|coauthors=and|month=(help) - ^ Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998 Jan-Feb;42(4):297-319. PMID 9493273
- ^ McMonnies CW, Boneham GC. Keratoconus, allergy, itch, eye-rubbing and hand-dominance. Clin Exp Optom. 2003 Nov;86(6):376-84. PMID 14632614
- ^ Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000 Aug;84(8):834-6. PMID 10906086
- ^ Jafri B, Lichter H, Stulting RD. Asymmetric keratoconus attributed to eye rubbing. Cornea. 2004 Aug;23(6):560-4. PMID 15256993
- ^ Ioannidis AS, Speedwell L, Nischal KK. Unilateral keratoconus in a child with chronic and persistent eye rubbing. Am J Ophthalmol. 2005 Feb;139(2):356-7. PMID 15734005
- ^ Lindsay RG, Bruce AS, Gutteridge IF. Keratoconus associated with continual eye rubbing due to punctal agenesis. Cornea. 2000 Jul;19(4):567-9. PMID 10928781
- ^ Rubinstein MP, Sud S. The use of hybrid lenses in management of the irregular cornea. Cont Lens Anterior Eye. 1999;22(3):87-90. PMID 16303411
- ^ Pullum KW, Buckley RJ. A study of 530 patients referred for rigid gas permeable scleral contact lens assessment. Cornea. 1997 Nov;16(6):612-22. PMID 9395869
- ^ Schirmbeck T, Paula JS, Martin LF, Crosio Filho H, Romao E. Efficacy and low cost in keratoconus treatment with rigid gas-permeable contact lens. Arq Bras Oftalmol. 2005 Mar-Apr;68(2):219-22. Epub 2005 May 18. PMID 15905944
- ^ a b Javadi MA, Motlagh BF, Jafarinasab MR, Rabbanikhah Z, Anissian A, Souri H, Yazdani S. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005 Nov;24(8):941-6. PMID 16227837
- ^ Mamalis N, Anderson CW, Kreisler KR, Lundergan MK, Olson RJ. Changing trends in the indications for penetrating keratoplasty. Arch Ophthalmol. 1992 Oct;110(10):1409-11. PMID 1417539
- ^ Al-Mezaine H, Wagoner MD. Repeat penetrating keratoplasty: indications, graft survival, and visual outcome. Br J Ophthalmol. 2006 Mar;90(3):324-7. PMID 16488955
- ^ Rubinfeld RS, Traboulsi EI, Arentsen JJ, Eagle RC Jr. Keratoconus after penetrating keratoplasty. Ophthalmic Surg. 1990 Jun;21(6):420-2. PMID 2381677
- ^ Wagoner MD, Smith SD, Rademaker WJ, Mahmood MA. Penetrating keratoplasty vs. epikeratoplasty for the surgical treatment of keratoconus. J Refract Surg. 2001 Mar-Apr;17(2):138-46. PMID 11310764
- ^ US FDA, New Humanitarian Device Approval INTACS® Prescription Inserts for Keratoconus - H040002
- ^ Simon G, Parel JM, Lee W, Kervick GN. Gel injection adjustable keratoplasty. Graefes Arch Clin Exp Ophthalmol. 1991;229(5):418-24. PMID 1718824
- ^ Ruckhofer J. Clinical and histological studies on the intrastromal corneal ring segments (ICRS®, Intacs®) Klin Monatsbl Augenheilkd. 2002 Aug;219(8):555-6. PMID 12353173
- ^ a b Miranda D, Sartori M, Francesconi C, Allemann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. J Refract Surg. 2003 Nov-Dec;19(6):645-53. PMID 14640429
- ^ Colin J, Velou S. Current surgical options for keratoconus., J Cataract Refract Surg. 2003 Feb;29(2):379-86. PMID 12648653
- ^ Bergmanson JP, Farmer EJ. A return to primitive practice? Radial keratotomy revisited. Cont Lens Anterior Eye. 1999;22(1):2-10. PMID 16303397
- ^ a b Lombardi M, Abbondanza M Asymmetric radial keratotomy for the correction of keratoconus. J Refract Surg. 1997 May-Jun;13(3):302-7. PMID 9183763
External links
Keratoconus associations:
- KC Support
- Keratoconus Australia
- National Keratoconus Foundation, USA
- Keratoconus Self Help and Support Group, UK
- International Keratoconus Support Forum
Web articles on keratoconus:
Individuals' experiences with keratoconus:
- Julia Ziobro's Cornea Transplant Diary, right eye, left eye
- John Ackerman's transplant diary and keratoconus resources
- Mandy Patinkin, actor, describes his corneal transplant
- Patient-owned database on Mini Asymmetric radial keratotomy
Other:
