Insect wing

|

|
| Original veins and wing posture of a dragonfly. | Hoverflies hovering to mate. |
| File:Lepidoptera wing.jpg | 
|
| Cutout of a butterfly's wing with magnification. | Hardened forewings and hind wings unfolding in a beetle. |
Insects are the only group of invertebrates known to have evolved flight. Insects possess some remarkable flight characteristics and abilities, still far superior to attempts by humans to replicate their capabilities. Even our understanding of the aerodynamics of flexible, flapping wings and how insects fly is imperfect. One application of this research is in the engineering of extremely small micro air vehicles with low Reynolds numbers. Insect wings are adult outgrowths of the insect exoskeleton that enable insects to fly. They are found on the second and third thoracic segments (the mesothorax and metathorax), and the two pairs are often referred to as the forewings and hindwings, respectively, though a few insects lack hindwings, even rudiments. Insect wings do not constitute appendages in technical parlance, as insects only have one pair of appendages per segment. The wings are strengthened by a number of longitudinal veins, which often have cross-connections that form closed "cells" in the membrane (extreme examples include Odonata and Neuroptera). The patterns resulting from the fusion and cross-connection of the wing veins are often diagnostic for different evolutionary lineages and can be used for identification to the family or even genus level in many orders of insects.
The physical dynamics of flight are composed of direct and indirect flight. Those species that employ direct flight have wing muscles directly attached the wing base so that a small movement of the wing base downward, lifts the wing itself upward. However Insects with indirect flight have muscles that attach to thorax and deform it; since the wings are extensions of the thoracic exoskeleton, the deformations of the thorax cause the wings to move as well.
The wings may be present in only one sex (often the male) in some groups such as velvet ants and Strepsiptera, or selectively lost in "workers" of social insects such as ants and termites. Rarely, the female is winged but the male not, as in fig wasps. In some cases, wings are produced only at particular times in the life cycle, such as in the dispersal phase of aphids. Beyond the mere presence/absence of wings, the structure and colouration will often vary with morphs, such as in the aphids, migratory phases of locusts and in polymorphic butterflies.
At rest, the wings may be held flat, or folded a number of times along specific patterns; most typically, it is the hindwings which are folded, but in a very few groups such as vespid wasps, it is the forewings. How and why insect wings evolved is not well understood. Three main theories on the origins of insect flight are that wings developed from paranotal lobes, extensions of the thoracic terga; that they are modifications of movable abdominal gills as found on aquatic naiads of mayflies and that insect wings arose from the fusion of pre-existing endite and exite structures each with pre-existing articulation and tracheation.
Morphology
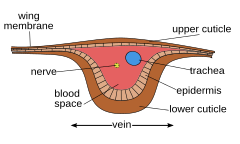
Internal
Each of the wings consists of a thin membrane supported by a system of veins. The membrane is formed by two layers of integument closely apposed, while the veins are formed where the two layers remain separate and the cuticle may be thicker and more heavily sclerotized. Within each of the major veins is a nerve and a trachea, and, since the cavities of the veins are connected with the hemocoel, hemolymph can flow into the wings.[1] Also are the wings lumen, being an extension of the hemocoel, which contains the tracheae, nerves, and hemolymph. As the wing develops, the dorsal and ventral integumental layers become closely apposed over most of their area forming the wing membrane. The remaining areas form channels, the future veins, in which the nerves and tracheae may occur. The cuticle surrounding the veins becomes thickened and more heavily sclerotized to provide strength and rigidity to the wing. Hairs of two types may occur on the wings: microtrichia, which are small and irregularly scattered, and macrotrichia, which are larger, socketed, and may be restricted to veins. The scales of Lepidoptera and Trichoptera are highly modified macrotrichia.[2]
Veins

In some very small insects, the venation may be greatly reduced. In Chalcidoidea (Chalcid wasps), for instance, only the subcosta and part of the radius are present. Conversely, an increase in venation may occur by the branching of existing veins to produce accessory veins or by the development of additional, intercalary veins between the original ones, as in the wings of Orthoptera (grasshoppers and crickets). Large numbers of cross-veins are present in some insects, and they may form a reticulum as in the wings of Odonata (dragonflies and damselflies) and at the base of the forewings of Tettigonioidea and Acridoidea (katydids and grasshoppers respectively).[1]
The archedictyon is the name given to a hypothetical scheme of wing venation proposed for the very first winged insect. It is based on a combination of speculation and fossil data. Since all winged insects are believed to have evolved from a common ancestor, the archediction represents the "template" that has been modified (and streamlined) by natural selection for 200 million years. According to current dogma, the archedictyon contained 6–8 longitudinal veins. These veins (and their branches) are named according to a system devised by John Comstock and George Needham—the Comstock-Needham System:[3]
- Costa (C) – the leading edge of the wing
- Subcosta (Sc) – second longitudinal vein (behind the costa), typically unbranched
- Radius (R) – third longitudinal vein, one to five branches reach the wing margin
- Media (M) – fourth longitudinal vein, one to four branches reach the wing margin
- Cubitus (Cu) – fifth longitudinal vein, one to three branches reach the wing margin
- Anal veins (A1, A2, A3) – unbranched veins behind the cubitus
The costa (C) is the leading marginal vein on most insects, although sometimes there is a small vein above the costa called the precosta, although in almost all extant insects,[4]: 41–42 the precosta is fused with the costa; The costa rarely ever branches because is at the leading edge, which is associated at its base with the humeral plate. The trachea of the costal vein is perhaps a branch of the subcostal trachea. Located after the costa is the third vein, the subcosta, which branches into two two separate veins: the anterior and posterior. The base of the subcosta is associated with the distal end of the neck of the first axillary (see section below). The fourth vein is the radius (R), which is branched into five separate veins. The radius is generally the strongest vein of the wing. Toward the middle of the wing, it forks into a first undivided branch (R1) and a second branch, called the radial sector (Ra), which subdivides dichotomously into four distal branches (R2, R3, R4, R5). Basally, the radius is flexibly united with the anterior end of the second axillary (2Ax).[5]
The fifth vein of the wing is the media. In the archetype pattern (A), the media forks into two main branches: a media anterior (MA), which divides into two distal branches (MA1, MA2), and a median sector, or media posterior (MP), which has four terminal branches (M1, M2, M3, M4). In most modern insects the media anterior has been lost, and the usual "media" is the four-branched media posterior with the common basal stem. In the Ephemerida, according to present interpretations of the wing venation, both branches of the media are retained, while in Odonata the persisting media is the primitive anterior branch. The stem of the media is often united with the radius, but when it occurs as a distinct vein its base is associated with the distal median plate (m') or is continuously sclerotized with the latter. The cubitus, the sixth vein of the wing, is primarily two branched. The primary forking of the takes place near the base of the wing, forming the two principal branches (Cu1, Cu2). The anterior branch may break up into a number of secondary branches, but commonly it forks into two distal branches. The second branch of the cubitus (Cu2) in Hymenoptera, Trichoptera, and Lepidoptera was mistaken by Comstock and Needham for the first anal. Proximally the main stem of the cubitus is associated with the distal median plate (m') of the wing base.[5]
Postcubitus (Pcu) is the first anal of the Comstock and Needham system. The Postcubitus, however, has the status of an independent wing vein and should be recognized as such. In nymphal wings, its trachea arises between the cubital trachea and the group of vannal tracheae. In the mature wings of more generalized insect the Postcubitus is always associated proximally with the cubitus and is never intimately connected with the flexor sclerite (3Ax) of the wing base. In Neuroptera, Mecoptera, and Trichoptera the postcubitus may be more closely associated with the vannal veins, but its base is always free from the latter. The postcubitus is usually unbranched; it is primitively two branched. The vannal veins (lV to nV) are the anal veins that are immediately associated with the third axillary, and which are directly affected by the movement of this sclerite that brings about the flexion of the wings. In number the vannal veins vary. from 1 to 12, according to the expansion of the vannal area of the wing. The vannal tracheae usually arise from a common tracheal stem in nymphal insects, and the veins are regarded as branches of a single anal vein. Distally the vannal veins are either simple or branched. Jugal Veins (J) of the jugal lobe of the wing is often occupied by a network of irregular veins, or it may be entirely membranous; but sometimes it contains one or two distinct small veins, the first jugal vein, or vena arcuata, and the second jugal vein, or vena cardinalis (2J).[5]
- C-Sc cross-veins - run between the costa and subcosta
- R cross-veins - run between adjacent branches of the radius
- R-M cross-veins - run between the radius and media
- M-Cu cross-veins - run between the media and cubitus
All the veins of the wing are subject to secondary forking and to union by cross-veins. In some orders of insects the cross-veins are so numerous that the whole venational pattern becomes a close network of branching veins and cross-veins. Ordinarily, however, there is a definite number of cross-veins having specific locations. The more constant cross-veins are the humeral cross-vein (h) between costa and subcosta, the radial cross-vein (r) between R and the first fork of Rs, the sectorial cross-vein (s) between the two forks of R8, the median cross-vein (m-m) between M2 and M3, and the mediocubital cross-vein (m-cu) between media and cubitus.[5]
The veins of insect wings are characterized by a convex-concave placement, such as those seen in mayflies (i.e., concave is "down" and convex is "up") which alternate regularly and by it's triadic type of branching; whenever a vein forks there is always an interpolated vein of the opposite position between the two branches. A concave vein will fork into two concave veins (with the interpolated vein being convex) and the regular alteration of the veins is preserved.[6] The veins of the wing appear to fall into an undulating pattern according to whether they have a tendency to fold up or down when the wing is relaxed. The basal shafts of the veins are convex, but each vein forks distally into an anterior convex branch and a posterior concave branch. Thus the costa and subcosta are regarded as convex and concave branches of a primary first vein, Rs is the concave branch of the radius, posterior media the concave branch of the media, Cu1 and Cu2 are respectively convex and concave, while the primitive Postcubitus and the first vannal have each an anterior convex branch and a posterior concave branch. The convex or concave nature of the veins has been used as evidence in determining the identities of the persisting distal branches of the veins of modern insects, but it has not been demonstrated to be consistent for all wings.[1][5]
Fields
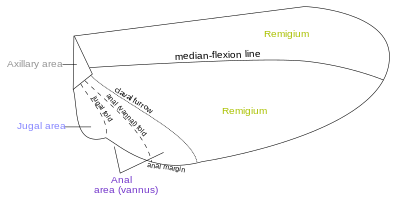
Wing areas are delimited and subdivided by fold-lines along which the wing can fold, and flexion-lines along which the wing can flex during flight. The fundamental distinction between the flexion-lines and the fold-lines is often blurred, as fold-lines may permit some flexibility or vice-versa. Two constants that are found in nearly all insect wings are the claval (a flexion-line) and jugal folds (or fold line); forming variable and unsatisfactory boundaries. Wing foldings can very complicated, with transverse folding occurs in the hind wings of Dermaptera and Coleoptera, and in some insects the anal area can be folded like a fan.[4] There are about four different fields found on the insect wings:
- Remigium
- Anal area (vannus)
- Jugal area
- Axillary area
- Alula
Most veins and crossveins occur in the anterior area of the remigium, which is responsible for most of the flight, powered by the thoracic muscles. The posterior portion of the remigium is sometimes called the clavus; the two other posterior fields are the anal and jugal ares.[4] When the vannal fold has the usual position anterior to the group of anal veins, the remigium contains the costal, subcostal, radial, medial, cubital, and postcubital veins. In the flexed wing the remigiumturns posteriorly on the flexible basal connection of the radius with the second axillary, and the base of the mediocubital field is folded medially on the axillary region along the plica basalis (bf) between the median plates (m, m') of the wing base.[5]
The vannus is bordered by the vannal fold, which typically occurs between the postcubitus and the first vannal vein. In Orthoptera it usually has this position. In the forewing of Blattidae, however, the only fold in this part of the wing lies immediately before the postcubitus. In Plecoptera the vannal fold is posterior to the postcubitus, but proximally it crosses the base of the first vannal vein. In the cicada the vannal fold lies immediately behind the first vannal vein (lV). These small variations in the actual position of the vannal fold, however, do not affect the unity of action of the vannal veins, controlled by the flexor sclerite (3Ax), in the flexion of the wing. In the hind wings of most Orthoptera a secondary vena dividens forms a rib in the vannal fold. The vannus is usually triangular in shape, and its veins typically spread out from the third axillary like the ribs of a fan. Some of the vannal veins may be branched, and secondary veins may alternate with the primary veins. The vannal region is usually best developed in the hind wing, in which it may be enlarged to form a sustaining surface, as in Plecoptera and Orthoptera. The great fanlike expansions of the hind wings of Acrididae are clearly the vannal regions, since their veins are all supported on the third axillary sclerites on the wing bases, though Martynov (1925) ascribes most of the fan areas in Acrididae to the jugal regions of the wings. The true jugum of the acridid wing is represented only by the small membrane (Ju) mesad of the last vannal vein. The jugum is more highly developed in some other Orthoptera, as in the Mantidae. In most of the higher insects with narrow wings the vannus becomes reduced, and the vannal fold is lost, but even in such cases the flexed wing may bend along a line between the postcubitus and the first vannal vein.[5]
The Jugal Region, or Neala, is a region of the wing that is usually a small membranous area proximal to the base of the vannus strengthened by a few small, irregular veinlike thickenings; but when well developed it is a distinct section of the wing and may contain one or two jugal veins. When the jugal area of the forewing is developed as a free lobe, it projects beneath the humeral angle of the hind wing and thus serves to yoke the two wings together. In the Jugatae group of Lepidoptera it bears a long finger-like lobe. The jugal region was termed the neala ("new wing") because it is evidently a secondary and recently developed part of the wing.[5]
The axilary region is region containing the axillary sclerites has in general the form of a scalene triangle. The base of the triangle (a-b) is the hinge of the wing with the body; the apex (c) is the distal end of the third axillary sclerite; the longer side is anterior to the apex. The point d on the anterior side of the triangle marks the articulation of the radial vein with the second axillary sclerite. The line between d and c is the plica basalis (bf), or fold of the wing at the base of the mediocubital field.[5]
At the posterior angle of the wing base in some Diptera there is a pair of membranous lobes (squamae, or calypteres) known as the alula. The alula is well developed in the house fly. The outer squama (c) arises from the wing base behind the third axillary sclerite (3Ax) and evidently represents the jugal lobe of other insects (A, D); the larger inner squama (d) arises from the posterior scutellar margin of the tergum of the wing-bearing segment and forms a protective, hoodlike canopy over the halter. In the flexed wing the outer squama of the alula is turned upside down above the inner squama, the latter not being affected by the movement of the wing. In many Diptera a deep incision of the anal area of the wing membrane behind the single vannal vein sets off a proximal alar lobe distal to the outer squama of the alula.[5]
Joints

The various movements of the wings, especially in insects that flex the wings horizontally over the back when at rest, which demands a more complicated articular structure at the wing base than a mere hinge of the wing with the body. Each wing is attached to the body by a membranous basal area, but the articular membrane contains a number of small articular sclerites, collectively known as the pteralia. The pteralia include an anterior humeral plate at the base of the costal vein, a group of axillaries (Ax) associated with the subcostal, radial, and vannal veins, and two less definite median plates (m, m') at the base of the mediocubital area. The axillaries are specifically developed only in the wing-flexing insects, where they constitute the flexor mechanism of the wing operated by the flexor muscle arising on the pleuron. Characteristic of the wing base is also a small lobe on the anterior margin of the articular area proximal to the humeral plate, which, in the forewing of some insects, is developed into a large, flat, scale-like flap, the tegula, overlapping the base of the wing. Posteriorly the articular membrane often forms an ample lobe between the wing and the body, and its margin is generally thickened and corrugated, giving the appearance of a ligament, the so-called axillary cord, continuous mesally with the posterior marginal scutellar fold of the tergal plate bearing the wing.[5]
The articular sclerites, or pteralia, of the wing base of the wing-flexing insects and their relations to the body and the wing veins, shown diagrammatically, are as follows:
- Humeral plates
- First Axillary
- Second Axillary
- Third Axillary
- Fourth Axillary
- Median plates (m, m')
The humeral plate is usually a small sclerite on the anterior margin of the wing base, movable and articulated with the base of the costal vein. Odonata have their humeral plate greatly enlarged,[5] with two muscles arising from the episternum inserted into the Humeral plates and two from the edge of the epimeron inserted into the axillary plate.[1]
The first axillary sclerite (lAx) is the anterior hinge plate of the wing base. Its anterior part is supported on the anterior notal wing process of the tergum (ANP); its posterior part articulates with the tergal margin. The anterior end of the sclerite is generally produced as a slender arm, the apex of which (e) is always associated with the base of the subcostal vein (Sc), though it is not united with the latter. The body of the sclerite articulates laterally with the second axillary. The second axillary sclerite (2Ax) is more variable in form than the first axillary, but its mechanical relations are no less definite. It is obliquely hinged to the outer margin of the body of the first axillary, and the radial vein (R) is always flexibly attached to its anterior end (d). The second axillary presents both a dorsal and a ventral sclerotization in the wing base; its ventral surface rests upon the fulcral wing process of the pleuron. The second axillary, therefore, is the pivotal sclerite of the wing base, and it specifically manipulates the radial vein.[5]
The third axillary sclerite (3Ax) lies in the posterior part of the articular region of the wing. Its form is highly variable and often irregular, but the third axillary is the sclerite on which is inserted the flexor muscle of the wing (D). Mesally it articulates anteriorly (f) with the posterior end of the second axillary, and posteriorly (b) with the posterior wing process of the tergum (PNP), or with a small fourth axillary when the latter is present. Distally the third axillary is prolonged in a process which is always associated with the bases of the group of veins in the anal region of the wing here termed the vannal veins (V). The third axillary, therefore, is usually the posterior hinge plate of the wing base and is the active sclerite of the flexor mechanism, which directly manipulates the vannal veins. The contraction of the flexor muscle (D) revolves the third axillary on its mesal articulations (b, f) and thereby lifts its distal arm; this movement produces the flexion of the wing. The Fourth Axillary sclerite is not a constant element of the wing base. When present it is usually a small plate intervening between the third axillary and the posterior notal wing process and is probably a detached piece of the latter.[5]
The median plates (m, m') are also sclerites that are not so definitely differentiated as specific plates as are the three principal axillaries, but nevertheless they are important elements of the flexor apparatus. They lie in the median area of the wing base distal to the second and third axillaries and are separated from each other by an oblique line (bf) which forms a prominent convex fold during flexion of the wing. The proximal plate (m) is usually attached to the distal arm of the third axillary and perhaps should be regarded as a part of the latter. The distal plate (m') is less constantly present as a distinct sclerite and may be represented by a general sclerotization of the base of the mediocubital field of the wing. When the veins of this region are distinct at their bases, they are associated with the outer median plate.[5]
Muscles
The muscles that control flight in insects can take up to 10% to 30% of the total body mass. The muscles that control flight vary with the two types of flight found in insects: indirect and direct. Insects that use first, indirect, have the muscles attach to the tergum instead of the wings, as the name suggests. As the muscles contract the thoracic box becomes distorted, transferring the energy to the wing. There are two "bundles" muscles, those that span parallel to the tergum, the dorsolongitudinals, and those that are attached to the tegum and extend to the sternum, the dorsoventrals.[7] In direct muscle, the connection is directly from the pleuron (thoracic wall) to individual sclerites located at the base of the wing. The subalar and basalar muscles have ligament attachments to the subalar and basalar sclerites. Here resilin, a highly elastic material, forms the ligaments connecting flight muscles to the wing apparatus. Resilin is 100 times greater energy storage capabilities than muscle, and is therefore more efficient.[7]
In more derived orders of insects, such as Diptera (flies) and Hymenoptera (wasp), the indirect muscles occupy the greatest volume of the pterothorax and function as the primary source of power for the wingstroke. Contraction of the dorsolongitudinal muscles causes the severe arching of the notum which depresses the wing while contraction of the dorsoventral muscles causes opposite motion of notum. Other more primitive insects, such as Orthoptera (locusts), Coleoptera (beetles), and Odonata (dragonflies) use direct muscles that are responsible for developing the needed power for the up and down strokes.[7][8]
Insect wing muscle is a strictly aerobic tissue. Per unit protein it consumes fuel and oxygen at rates taking place in a very concencentrated and highly organized tissue so that the steady-state rates per unit volume represent an absolute record in biology. The fuel and oxygen rich blood is carried to the muscles through diffusion occurring in large amounts, in order to maintain the high level of energy used during flight. Many wing muscles are large and may be as large as 10 mm in length and 2 mm. in width. Moreover, in some Diptera the fibres are of giant dimensions. For instance, in the very active Rutilia, the cross-section is 1800 µm long and more than 500 µm wide. The transport of fuel and oxygen from the surroundings to the sites of consumption and the reverse transport of carbon dioxide therefore represent a challenge to the biologist both in relation to transport in the liquid phase and in the intricate system of air tubes, i.e. in the tracheal system[9]
Coupling, folding, and other features
In many insect species, the fore and hind wing are coupled together, which improves the aerodynamic efficiency of flight. The most common coupling mechanism (e.g., Hymenoptera and Trichoptera) is a row of small hooks on the forward margin of the hind wing, or "hamuli", which lock onto the fore wing, keeping them held together (hamulate coupling). In some other insect species (e.g., Mecoptera, Lepidoptera, and some Trichoptera) the jugal lobe of the fore wing covers a portion of the hind wing (jugal coupling), or the margins of the fore and hind wing overlap broadly (amplexiform coupling), or the hindwing bristles, or frenulum, hook under the retaining structure or retinaculum on the forewing.[4]: 43
When at rest, the wings are held over the back in most insects, which may involve longitudinal folding of the wing membrane and sometimes also transverse folding. Folding may sometimes occur along the flexion lines. Though fold lines may be transverse, as in the hind wings of beetles and earwigs, they are normally radial to the base of the wing, allowing adjacent sections of a wing to be folded over or under each other. The commonest fold line is the jugal fold, situated just behind the third anal vein,[2] although, most Neoptera have a jugal fold just behind vein 3A on the forewings. It is sometimes also present on the hindwings. Where the anal area of the hindwing is large, as in Orthoptera and Blattodea, the whole of this part may be folded under the anterior part of the wing along a vannal fold a little posterior to the claval furrow. In addition, in Orthoptera and Blattodea, the anal area is folded like a fan along the veins, the anal veins being convex, at the crests of the folds, and the accessory veins concave. Whereas the claval furrow and jugal fold are probably homologous in different species, the vannal fold varies in position in different taxa. Folding is produced by a muscle arising on the pleuron and inserted into the third axillary sclerite in such a waythat, when it contracts, the sclerite pivots about its points of articulation with the posterior notal process and the second axillary sclerite.[1]
As a result, the distal arm of the third axillary sclerite rotates upwards and inwards, so that finally its position is completely reversed. The anal veins are articulated with this sclerite in such a way that when it moves they are carried with it and become flexed over the back of the insect. Activity of the same muscle in flight affects the power output of the wing and so it is also important in flight control. In orthopteroid insects, the elasticity of the cuticle causes the vannal area of the wing to fold along the veins. Consequently energy is expended in unfolding this region when the wings are moved to the flight position. In general, wing extension probably results from the contraction of muscles attached to the basalar sclerite or, in some insects, to the subalar sclerite.[1]
Physiology
Biochemistry
The biochemistry of insect flight has been a focus of considerable study. While many insects use carbohydrates and lipids as the energy source for flight, many beetles and flies prefer to use the amino acid, proline, as their energy source.[10] Some species also use a combination of sources and moths such as Manduca sexta prefer to use carbohydrates for pre-flight warm-up.[11]
Insects that beat their wings less than one hundred times a second use synchronous muscle. A Synchronous muscle is a type of muscle that contracts once for every nerve impulse, which is more efficient for fast flight.[12] Insects that beat their wings more rapidly use asynchronous muscle; this is a type of muscle that contracts more than once per nerve impulse. This is achieved by the muscle being stimulated to contract again by a release in tension in the muscle, which can happen more rapidly than through simple nerve stimulation alone.[13]
Direct flight
Unlike most other insects, the wing muscles of species of Ephemeroptera (mayflies) and Odonata (dragonflies and damselflies) (the two living orders traditionally classified as "Paleoptera") insert directly at the wing bases, which are hinged so that a small movement of the wing base downward, lifts the wing itself upward, very much like rowing through the air. In mayflies, the hind wings are reduced, sometimes absent, and play little role in their flight, which is not particularly agile or graceful. In contrast, even though dragonflies cannot hover in still air with this primitive mechanism (although, with careful use of wind currents, they can remain nearly stationary), damselflies can, and in both groups, the fore and hind wings are similar in shape and size, and operated independently, which gives a degree of fine control and mobility in terms of the abruptness with which they can change direction and speed, not seen in other flying insects. This is not surprising, given that odonates are all aerial predators, and they have always hunted other airborne insects - evolutionary pressures have led to more advanced flight ability.[14]
Indirect flight
Other than the two orders with direct flight muscles, all other living winged insects fly using a different mechanism, involving indirect flight muscles. This mechanism evolved once, and is the defining feature (synapomorphy) for the infraclass Neoptera; it corresponds, probably not coincidentally, with the appearance of a wing-folding mechanism, which allows Neopteran insects to fold the wings back over the abdomen when at rest (though this ability has been lost secondarily in some groups, such as all butterflies).[14]
In the higher groups with two functional pairs of wings, both pairs are linked together mechanically in various ways, and function as a single wing, although this is not true in the more primitive groups. What all Neoptera share, however, is the way the muscles in the thorax work: these muscles, rather than attaching to the wings, attach to the thorax and deform it; since the wings are extensions of the thoracic exoskeleton, the deformations of the thorax cause the wings to move as well. A set of dorsal longitudinal muscles compress the thorax from front to back, causing the dorsal surface of the thorax (notum) to bow upward, making the wings flip down. A set of tergosternal muscles pull the notum downward again, causing the wings to flip upward. [14][15] In a few groups, the downstroke is accomplished solely through the elastic recoil of the thorax when the tergosternal muscles are relaxed. Several small sclerites at the wing base have other, separate, muscles attached and these are used for fine control of the wing base in such a way as to allow various adjustments in the tilt and amplitude of the wing beats. One of the final refinements that has appeared in some of the higher Neoptera (Coleoptera, Diptera, and Hymenoptera) is a type of muscular or neural control system whereby a single nerve impulse causes a muscle fiber to contract multiple times; this allows the frequency of wing beats to exceed the rate at which the nervous system can send impulses. This specialized form of muscle is termed asynchronous flight muscle. The overall effect is that many higher Neoptera can hover, fly backward, and perform other feats involving a degree of fine control that insects with direct flight muscles cannot achieve.[14]
Aerodynamics
There are two basic aerodynamic models of insect flight. Most insects use a method that creates a spiralling leading edge vortex. These flapping wings move through two basic half-strokes. The downstroke starts up and back and is plunged downward and forward. Then the wing is quickly flipped over (supination) so that the leading edge is pointed backward. The upstroke then pushes the wing upward and backward. Then the wing is flipped again (pronation) and another downstroke can occur. The frequency range in insects with synchronous flight muscles typically is 5 to 200 hertz (Hz). In those with asynchronous flight muscles, wing beat frequency may exceed 1000 Hz. When the insect is hovering, the two strokes take the same amount of time. A slower downstroke, however, provides thrust.[16][17]
Identification of major forces is critical to understanding insect flight. The first attempts to understand flapping wings assumed a quasi-steady state. This means that the air flow over the wing at any given time was assumed to be the same as how the flow would be over a non-flapping, steady-state wing at the same angle of attack. By dividing the flapping wing into a large number of motionless positions and then analyzing each position, it would be possible to create a timeline of the instantaneous forces on the wing at every moment. The calculated lift was found to be too small by a factor of three, so researchers realized that there must be unsteady phenomena providing aerodynamic forces. There were several developing analytical models attempting to approximate flow close to a flapping wing. Some researchers predicted force peaks at supination. With a dynamically scaled model of a fruit fly, these predicted forces later were confirmed. Others argued that the force peaks during supination and pronation are caused by an unknown rotational effect that fundamentally is different from the translational phenomena. There is some disagreement with this argument. Through computational fluid dynamics, some researchers argue that there is no rotational effect. They claim that the high forces are caused by an interaction with the wake shed by the previous stroke.[16][17]
Similar to the rotational effect mentioned above, the phenomena associated with flapping wings are not completely understood or agreed upon. Because every model is an approximation, different models leave out effects that are presumed to be negligible. For example, the Wagner effect says that circulation rises slowly to its steady-state due to viscosity when an inclined wing is accelerated from rest. This phenomenon would explain a lift value that is less than what is predicted. Typically, the case has been to find sources for the added lift. It has been argued that this effect is negligible for flow with a Reynolds number that is typical of insect flight. The Wagner effect was ignored, consciously, in at least one recent model.[17] One of the most important phenomena that occurs during insect flight is leading edge suction. This force is significant to the calculation of efficiency. The concept of leading edge suction first was put forth to describe vortex lift on sharp-edged delta wings. At high angles of attack, the flow separates over the leading edge, but reattaches before reaching the trailing edge. Within this bubble of separated flow is a vortex. Because the angle of attack is so high, a lot of momentum is transferred downward into the flow. These two features create a large amount of lift force as well as some additional drag. The important feature, however, is the lift. Because the flow has separated, yet it still provides large amounts of lift, this phenomenon is called stall delay. This effect was observed in flapping insect flight and it was proven to be capable of providing enough lift to account for the deficiency in the quasi-steady-state models. This effect is used by canoeists in a sculling draw stroke.[16][17]
All of the effects on a flapping wing may be reduced to three major sources of aerodynamic phenomena: the leading edge vortex, the steady-state aerodynamic forces on the wing, and the wing’s contact with its wake from previous strokes. The size of flying insects ranges from about 20 micrograms to about 3 grams. As insect body mass increases, wing area increases and wing beat frequency decreases. For larger insects, the Reynolds number (Re) may be as high as 10000. For smaller insects, it may be as low as 10. This means that viscous effects are much more important to the smaller insects, although the flow is still laminar, even in the largest fliers.[17][18]
Another interesting feature of insect flight is the body tilt. As flight speed increases, the insect body tends to tilt nose-down and become more horizontal. This reduces the frontal area and therefore, the body drag. Since drag also increases as forward velocity increases, the insect is making its flight more efficient as this efficiency becomes more necessary. Additionally, by changing the geometric angle of attack on the downstroke, the insect is able to keep its flight at an optimal efficiency through as many manoeuvres as possible. The development of general thrust is relatively small compared with lift forces. Lift forces may be more than three times the insect's weight, while thrust at even the highest speeds may be as low as 20% of the weight. This force is developed primarily through the less powerful upstroke of the flapping motion.[17][19]
The second method of flight, fling and clap, functions differently. In this process, the wings clap together above the insect's body and then fling apart. As they fling open, the air gets sucked in and creates a vortex over each wing. This bound vortex then moves across the wing and, in the clap, acts as the starting vortex for the other wing. By this effect, circulation and thus, lift are increased to the extent of being higher, in most cases, than the typical leading edge vortex effect. One of the reasons this method is not employed by more insects is the expected damage and wear to the wings caused by the repeated clapping. It is prevalent, however, among insects that are very small and experience low Reynolds numbers.[16][17]
Governing equations
A wing moving in fluids experiences a fluid force, which Follows the conventions found in aerodynamics, the force component normal to the direction of the far field flow relative to the wing is referred to as lift (L), and the force component in the opposite direction of the flow is drag (D). At the Reynolds numbers considered here, an appropriate force unit is 1/2(ρU2S), where ρ is the density of the fluid, S the wing area, and U the wing speed. The dimensionless forces are called lift (CL) and drag (CD) coefficients, that is:[16]
CL and CD are constants only if the flow is steady. A special class of objects such as airfoils may reach a steady state when it slices through the fluid at a small angle of attack. In this case, the inviscid flow around an airfoil can be approximated by a potential flow satisfying the no-penetration boundary condition. The Kutta-Joukowski theorem of a 2D airfoil further assumes that the flow leaves the sharp trailing edge smoothly, and this determines the total circulation around an airfoil. The corresponding lift is given by Bernoulli's principle (Blasius theorem):[16]
The flows around birds and insects can be considered incompressible: The Mach number, or speed while moving through air, is typically 1/300 and the wing frequency is about 10–103 Hz. Using the governing equation as the Navier-Stokes equation being subject to the no-slip boundary condition, the equation is:[16]
Where u(x, t) is the flow field, p the pressure, ρ the density of the fluid, ν the kinematic viscosity, ubd the velocity at the boundary, and us the velocity of the solid. By choosing a length scale, L, and velocity scale, U, the equation can be expressed in nondimensional form containing the Reynolds number, Re = UL/ν . There are two obvious differences between an insect wing and an airfoil: An insect wing is much smaller and it flaps. Using a dragonfly as an example, Its chord (c) is about 1 cm, its wing length (l) about 4 cm, and its wing frequency (f) about 40 Hz. The tip speed (u) is about 1 m/s, and the corresponding Reynolds number, Re = uc/ν about 103. At the smaller end, a Chalcid wasp has a wing length of about 0.5–0.7 mm and beats its wing at about 400 Hz. Its Reynolds number is about 25. The range of Reynolds number in insect flight is about 10 to 104, which lies in between the two limits that are convenient for theories: inviscid steady flows around an airfoil and Stokes flow experienced by a swimming bacterium. For this reason, this intermediate range is not well understood. On the other hand, it is perhaps the most ubiquitous regime among the things we see. Falling leaves and seeds, fishes, and birds all encounter unsteady flows similar to that seen around an insect.[16]
In addition to the Reynolds number, there are at least two other relevant dimensionless parameters. A wing has three velocity scales: the flapping velocity with respect to the body (u), the forward velocity of the body (U0), and the pitching velocity (Ωc). The ratios of them form two dimensionless variables, U0/u and Ωc/u, the former is often referred to as the advance ratio, and it is also related to the reduced frequency, fc/U0.[16]
If an insect wing is rigid, for example, a Drosophila wing is approximately so, its motion relative to a fixed body can be described by three variables: the position of the tip in spherical coordinates, (Θ(t),Φ(t)), and the pitching angle ψ(t), about the axis connecting the root and the tip. To estimate the aerodynamic forces based on blade-element analysis, it is also necessary to determine the angle of attack (α). The typical angle of attack at 70% wingspan ranges from 25◦ to 45◦ in hovering insects (15◦ in hummingbirds). Despite the wealth of data available for many insects, relatively few experiments report the time variation of α during a stroke. They include wind tunnel experiments of a tethered locust and a tethered fly, and free hovering flight of a fruit fly.[16]
Because they are relatively easy to measure, the wing-tip trajectories have been reported more frequently. For example, selecting only flight sequences that produced enough lift to support a weight, will show that the wing tip follows an elliptical shape. Noncrossing shapes were also reported for other insects. Regardless of their exact shapes, the plugging-down motion indicates that insects may use aerodynamic drag in addition to lift to support its weight.[16]
Hovering
| Wing beats[20] | |
|---|---|
| Honeybee | 250 bit/s |
| Housefly | 190bit/s |
| Bumblebee | 130 bit/s |
| Syrphis Fly | 120 bit/s |
| Hornet | 100 bit/s |
| Horsefly | 96 bit/s |
| Hummingbird Moth | 85 bit/s |
| Aeschnid Dragonfly | 38 bit/s |
| Scorpion Fly | 28 bit/s |
| Damselfly | 16 bit/s |
| Large White Butterfly | 12 bit/s |
| Wing speed[20] | |
|---|---|
| Aeschnid Dragonfly | 15.6 mph |
| Hornet | 12.8 mph |
| Hummingbird Moth | 11.1 mph |
| Horsefly | 8.8 mph |
| Syrphid Fly | 7.8 mph |
| Bumblebee | 6.4 mph |
| Honeybee | 5.7 mph |
| Housefly | 4.4 mph |
| Damselfly | 3.3 mph |
| Scorpion Fly | 1.1 mph |
Many insects can hover, or stay in one spot in the air, doing so by beating their wings rapidly. The ability to do so, though, is complex; requiring the use of sideways stabilization as well as the lift necessary to overcome the force of gravity. The lifting force is caused by the downward stroke of the wings. As the wings push down on the surrounding air, the result reaction force of the air on the wings force the insect up. The wings of most insects are designed so that during the upward stroke the force on the wing are small. Due to the fact that the upbeat and downbeat force the insect down and up respectively, the insect oscillates and winds up staying in the same position.[18]
The distance the insect falls between wingbeats depends on how rapidly its wings are beating. If the insect flaps its wings at a slow rate, the time interval during which the lifting force is zero is longer, and therefore the insect falls farther than if its wings were beating rapidly. One can calculate the wingbeat frequency necessary for the insect to maintain a given stability in its amplitude. To simplify the calculations, one must assume that the lifting force is at a finite constant value while the wings are moving down and that it is zero while the wings are moving up. During the time interval Δt of the upward wingbeat, the insect drops a distance h under the influence of gravity.[18]
The upward stroke then restores the insect to its original position. Typically, it may be required that the vertical position of the insect change by no more than 0.1 mm (i.e., h = 0.1 mm). The maximum allowable time for free fall is then [18]
Since the up movements and the down movements of the wings are about equal in duration, the period T for a complete up-and-down wing is twice Δr, that is,[18]
The frequency of the beats, f, meaning the number of wingbeats per second, is represented by the equation:[18]
In the examples used the frequency used is 110 bit/s, which is the typical frequency found in insects. Although butterflies have a much slower frequency with about 10 bit/s, which means that they can't hover. While other insect may be able to produce a frequency of 1000 bit/s. Restoring the insect to the vertical position must be during the downward stroke, the average upward force, Fav, must be equal to twice the weight of the insect. Note that since the upward force on the insect body is applied only for half the time, the average upward force on the insect is simply its weight.[18]
Power input
One can now compute the power required to maintain hovering by, considering again an insect with mass m 0.1 g, average force, Fav, applied by the two wings during the downward stroke is two times the weight. Because the pressure applied by the wings is uniformly distributed over the total wing area, that means one can assume the force generated by each wing acts through a single point at the midsection of the wings. During the downward stroke, the center of the wings traverses a vertical distance d.[18] The total work done by the insect during each downward stroke is the product of force and distance; that is,
If the wings swing through the beat at an angle of 70◦, then in the case presented for the insect with 1 cm long wings, d is 0.57 cm. Therefore, the work done during each stroke by the two wings is:[18]
After, the energy has to go somewhere; here, in the example used, the mass of the insect has to be raised 0.1 mm during each downstroke. The energy E required for this task is:[18]
This is a negligible fraction of the total energy expended which clearly, most of the energy is expended in other processes. A more detailed analysis of the problem shows that the work done by the wings is converted primarily into kinetic energy of the air that is accelerated by the downward stroke of the wings. The power is the amount of work done in 1 sec; in the insect used as an example, makes 110 downward strokes per second. Therefore, its power output P is, strokes per second, and that means its power output P is:[18]
Power output
In the calculation of the power used in hovering, the examples used neglected the kinetic energy of the moving wings. The wings of insects, light as they are, have a finite mass; therefore, as they move they possess kinetic energy. Because the wings are in rotary motion, the maximum kinetic energy during each wing stroke is:[18]
Here I is the moment of inertia of the wing and ωmax is the maximum angular velocity during the wing stroke. To obtain the moment of inertia for the wing, we will assume that the wing can be approximated by a thin rod pivoted at one end. The moment of inertia for the wing is then:[18]
Where l is the length of the wing (1 cm) and m is the mass of two wings, which may be typically 10−3 g. The maximum angular velocity, ωmax, can be calculated from the maximum linear velocity, νmax, at the center of the wing:[18]
During each stroke the center of the wings moves with an average linear velocity νav given by the distance d traversed by the center of the wing divided by the duration Δt of the wing stroke. From our previous example, d = 0.57 cm and Δt = 4.5×10−3 sec. Therefore:[18]
The velocity of the wings is zero both at the beginning and at the end of the wing stroke, meaning the maximum linear velocity is higher than the average velocity. If we assume that the velocity varies sinusoidally along the wing path, the maximum velocity is twice as high as the average velocity. Therefore, the maximum angular velocity is:[18]
And the kinetic energy therefore is:[18]
Since there are two wing strokes (the upstroke and downstroke) in each cycle of the wing movement, the kinetic energy is 2×43 = 86 erg. This is about as much energy as is consumed in hovering itself.[18]
Elasticity

Insects gain kinetic energy when they accelerate, which is of course provided by the muscles. When the wings begin to decelerate toward the end of the stroke, this energy must dissipate. During the downstroke, the kinetic energy is dissipated by the muscles themselves and is converted into heat (this heat is sometimes used to maintain core body temperature). Some insects are able to utilize the kinetic energy in the upward movement of the wings to aid in their flight. The wing joints of these insects contain a pad of elastic, rubber-like protein called resilin. During the upstroke of the wing, the resilin is stretched. The kinetic energy of the wing is converted into potential energy in the stretched resilin, which stores the energy much like a spring. When the wing moves down, this energy is released and aids in the downstroke.[18]
Using a few simplifying assumptions, we can calculate the amount of energy stored in the stretched resilin. Although the resilin is bent into a complex shape, the example given shows the calculation as its a straight rod of area A and length. Furthermore, we will assume that throughout the stretch the resilin obeys Hooke's law. This is not strictly true as the resilin is stretched by a considerable amount and therefore both the area and Young’s modulus change in the process of stretching. The energy E stored in the stretched resilin is:[18]
Here Y is the Young’s modulus for resilin, which has been measured to be 1.8×107 dyn/cm2. Typically, in an insect the size of a bee the volume of the resilin may be equivalent to a cylinder 2×10−2 cm long and 4×10−4 cm2 in area. In the example given, the length of the resilin rod is increased by 50% when stretched. That is, Δℓ is 10−2 cm. Therefore in this case the energy stored in the resilin of each wing is:[18]
The stored energy in the two wings is 36 erg, which is comparable to the kinetic energy in the upstroke of the wings. Experiments show that as much as 80% of the kinetic energy of the wing may be stored in the resilin.[18]
Gliding
Gliding is a form of flight, without the use of thrust. This form of flight is not common in insects, however there are a few species that do employ this unique ability. One of these includes some species of arboreal or tree dwelling ants, called Gliding ants (e.i., C. atratus, P. gracilis, C. heathi, D. armigerum, A. erinaceus). One of the most well known, and most studied, is the species is Cephalotes atratus. Rather than falling straight down however, the wingless tropical ant glides backwards as it falls, steering itself in a directed descent that lands it on the trunk of the tree; from which it fell—saving these insects from reaching the hostile and unfamiliar terrain of the forest floor. The ant uses its legs to slow its descent, then uses its elongated hind legs to steer their bodies while gliding, which rights itself early in their fall. However, all the legs are able to aid in the gliding process, shown in studies when removing certain legs.[21]
These arboreal ants locate the tree to which they land on based on its color, attracted by white or more brightly colored trunks, which closely resemble their natural targets: lichen-covered tree trunks. The benefit of this is that the insect will avoid being lost amongst the forest floor, which is the location of many of its natural predators. Day-active ants that foraging in the lowland rain forest canopy are frequently exposed to predators, such as lizards, birds, anteaters, and disturbance (e.g., passing animals and wind) in tree crowns. If a worker ant happens to drop off from its safe haven, it will most likely land on unfamiliar vegetation or on leaf litter in the understory. Leaf litter along with the rest of the forest floor is a particularly complex foreign terrain and harbors a variety of predators that are not found in the canopy.[21]
One of the only other examples known of insects gliding are found in arboreal bristletails (Archaeognatha), a kind of wingless arthropod. They were observed using the little barbs sticking out of their tails to guide their descent, thus increasing their chances of landing in a neighboring tree. When cutting these barbs off and dropped, the number of landings remarkably reduced.[21]
Evolution
Sometime in the Carboniferous Period, some 350 million years ago, when there were only two major land masses, insects began flying. How and why insect wings developed, however, is not well understood, largely due to the scarcity of appropriate fossils from the period of their development in the Lower Carboniferous. Three main theories on the origins of insect flight are that wings developed from paranotal lobes, extensions of the thoracic terga; that they are modifications of movable abdominal gills as found on aquatic naiads of mayflies; or that they developed from thoracic protrusions used as radiators.[22]
Fossils

Fossils from the Devonian (400 million years ago) are all wingless, but by the Carboniferous (320 million years ago), more than 10 different genera of insects had fully functional wings. There is little preservation of transitional forms between the two periods. The earliest winged insects are from this time period (Pterygota), including the aforementioned Blattaria, Caloneurodea, primitive stem-group Ephemeropterans, Orthoptera and Palaeodictyopteroidea. Very early Blattarians---during the Carboniferous---had a very large discoid pronotum and coriaceous forewings with a distinct CuP vein (a unbranched wing vein, lying near the claval fold and reaching the wing posterior margin).[23]: 399 Even though the oldest definitive insect fossil is the Devonian Rhyniognatha hirsti, estimated at 396-407 million years old, it possessed dicondylic mandibles, a feature associated with winged insects.[24]
During the Permian, the dragonflies Odonata were the dominant aerial predator and probably dominated terrestrial insect predation as well. True Odonata appeared in the Permian[25][26] and all are amphibian. Their prototypes are the oldest winged fossils,[27] go back to the Devonian, and are different from other wings in every way.[28] Their prototypes may have had the beginnings of many modern attributes even by late Carboniferous and it is possible that they even captured small vertebrates, for some species had a wing span of 71 cm.[29] The earliest beetle-like species during the Permian had pointed, leather like forewings with cells and pits. Hemiptera, or true bugs had appeared in the form of Arctiniscytina and Paraknightia having forewings with unusual venation, possibly diverging from Blattoptera.[23]: 186
A single large wing from a species of Diptera in the Triassic (10 mm instead of usual 2–6 mm) was found in Australia (Mt. Crosby).This family Tilliardipteridae, despite of the numerous 'tipuloid' features, should be included in Psychodomorpha sensu Hennig on account of loss of the convex distal 1A reaching wing margin and formation of the anal loop.[30]
Theories
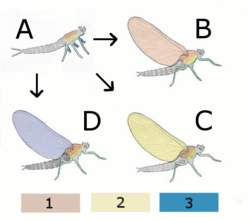
A Hypothetical wingless ancestor
B Paranotal theory:
Hypothetical insect with wings from the back (Notum)
C Hypothetical insect with wings from page (Pleurum)
D Epicoxal theory
Hypothetical insect with wings from Annex of the legs
1 Notum (back)
2 Pleurum (page)
3 Exit (outer attachments of the legs)
- Paranotal hypothesis: This hypothesis suggests that the insect's wings developed from paranotal lobes, a preadaptation found in insect fossils that is believed to have assisted stabilization while hopping or falling. In favor of this hypothesis is the tendency of most insects, when startled while climbing on branches, to escape by dropping to the ground. Such lobes would have served as parachutes and enable the insect to land more softly. The theory suggests that these lobes gradually grew larger and in a later stage developed a joint with the thorax. Even later would appear the muscles to move these crude wings. This model implies a progressive increase in the effectiveness of the wings, starting with parachuting, then gliding and finally active flight. Still, lack of substantial fossil evidence of the development of the wing joints and muscles poses a major difficulty to the theory, as does the seemingly spontaneous development of articulation and venation, and it has been largely rejected by experts in the field.[22]
- Epicoxal hypothesis:This theory suggested that a possible origin for insect wings might have been the movable abdominal gills found in many aquatic insects, such as on naiads of mayflies. According to this theory these tracheal gills, which started their way as exits of the respiratory system and over time were modified into locomotive purposes, eventually developed into wings. The tracheal gills are equipped with little winglets that perpetually vibrate and have their own tiny straight muscles.[22]
- Endite-exite hypothesis:The hypothesis with perhaps the strongest evidence is that which stems from the adaptation of endites and exites, appendages on the respective inner and outer aspects of the primitive arthropod limb. This was advanced by Trueman[31] based on a study by Goldschmidt in 1945 on Drosophila melanogaster, in which a pod variation displayed a mutation transforming normal wings to what was interpreted as a triple-jointed leg arrangement with some additional appendages but lacking the tarsus, where the wing's costal surface normally would be. This mutation was reinterpreted as strong evidence for a dorsal exite and endite fusion, rather than a leg, with the appendages fitting in much better with this hypothesis. The innervation, articulation and musculature required for the evolution of wings are already present in podomeres.[22]
- Paranota plus leg gene recruitment hypothesis:The fossil larvae of Coxoplectoptera provided important new clues to the disputed question of the evolutionary origin of insect wings. Before the larvae fossil discovery the paranotal-hypothesis and the leg-exite-hypothesis have been considered as incompatible alternative explanations, which have both been supported by a set of evidences from the fossil record, comparative morphology, developmental biology and genetics. The expression of leg genes in the ontogeny of the insect wing has been universally considered as conclusive evidence in favour of the leg-exite-hypothesis, which proposes that insect wings are derived from mobile leg appendages (exites). However, the larvae of Coxoplectoptera show that the abdominal gills of mayflies and their ancestors, which are generally considered as corresponding structures to insect wings, articulated within the dorsal tergite plates. This cannot be seen in modern mayfly larvae, because their abdominal tergites and sternites are fused to rings, without any traces left even in embryonic development. If larval gills and wings are corresponding ("serial homologous") structures and thus share the same evolutionary origin, the new results from Coxoplectoptera demonstrate that also wings are of tergal origin, as proposed by the classical paranotal-hypothesis. Staniczek, Bechly & Godunko (2011)[22][32] therefore suggested a new hypothesis that could reconcile the apparently conflicting evidence from paleontology and developmental genetics: wings first originated as stiff outgrowths of tergal plates (paranota), and only later in evolution became mobile, articulated appendages through secondary recruiting of leg genes.[22]
Suggestions have been made that wings may have evolved initially for sailing on the surface of water as seen in some stoneflies.[33] An alternative idea is that it drives from directed aerial gliding descent—a preflight phenomena found in some apterygote, a wingless sister taxa to the winged insects.[34] The earliest fliers were similar to dragonflies with two sets of wings, direct flight muscles, and no ability to fold their wings over their abdomens. Most insects today, which evolved from those first fliers, have simplified to either one pair of wings or two pairs functioning as a single pair and using a system of indirect flight muscles.[22]
Natural selection has played an enormous role in refining the wings, control and sensory systems, and anything else that affects aerodynamics or kinematics. One noteworthy trait is wing twist. Most insect wings are twisted, as are helicopter blades, with a higher angle of attack at the base. The twist generally is between 10 and 20 degrees. In addition to this twist, the wing surfaces are not necessarily flat or featureless; most larger insects have wing membranes distorted and angled between the veins in such a way that the cross-section of the wings approximates an airfoil. Thus, the wing's basic shape already is capable of generating a small amount of lift at zero angle of attack (see Insect wing). Most insects control their wings by adjusting tilt, stiffness, and flapping frequency of the wings with tiny muscles in the thorax (below). Some insects evolved other wing features that are not advantageous for flight, but play a role in something else, such as mating or protection.[22]
| Evolution of the ways the wings at rest to the body to create | ||
| wings do not fold back (recent Archaeoptera) |
spread laterally (large bubbles) | |
| over the back against one another (damselflies, mayflies) | ||
| Folding (Neoptera) | ||
| wings not foldable (e.g., stoneflies) | ||
| Folding | fan-fold (e.g., front wings of wasps) | |
| Cross fold (such as the rear wing of the beetle) | ||
| Subjects folding (such as the rear wing of the earwigs) | ||
Some insects, occupying the biological niches that they do, need to be incredibly maneuverable. They must find their food in tight spaces and be capable of escaping larger predators - or they may themselves be predators, and need to capture prey. Their maneuverability, from an aerodynamic viewpoint, is provided by high lift and thrust forces. Typical insect fliers can attain lift forces up to three times their weight and horizontal thrust forces up to five times their weight. There are two substantially different insect flight mechanisms, and each has its own advantages and disadvantages - just because odonates have a more primitive flight mechanism does not mean they are less able fliers; they are, in certain ways, more agile than anything that has evolved afterward.[22]
Morphogenesis
While the development of wings in insects is clearly defined in those who are members of Endopterygota, which undergo complete metamorphosis; in these species, the wing develops while in the pupal stage of the insects life cycle. However, insects that undergo incomplete metamorphosis do not have a pupal stage, therefore they must have a different wing morphogenesis. Insects such as those that are hemimetabolic have wings that start out as buds, which are found underneath the exoskeleton, and do not become exposed to the last instar of the nymph.[35]
The first indication of the wing buds is of a thickening of the hypodermis, which can be observed in insect species as early the embryo, and in the earliest stages of the life cycle. During the development of morphological features while in the embryo, or embryogenesis, a cluster of cells grow underneath the ectoderm which later in development, after the lateral ectoderm has grown dorsally to form wind imaginal disc. An example of wing bud development in the larvae, can be seen in those of White butterflies (Pieris). In the second instar the histoblast become more prominent, which now form a pocket-like structure. As of the third and fourth instars, the histoblast become more elongated. This greatly extended and evaginated, or protruding, part is what becomes the wing. By the close of the last instar, or fifth, the wing is pushed out of the wing-pocket, although continues to lie under the old larval cuticle while in its prepupal stage. It is not until the butterfly is in its pupal stage that the wing-bud becomes exposed, and shortly after eclosion, the wing begins to expand and form its definitive shape.[35]
The development of tracheation of the wings begin before the wing histoblast form, as it is important to note that they develop near a large trachea. During the fourth instar, cells from the epithelium of this trachea become greatly enlarged extend into the cavity of the wing bud, with each cell having developed a closely coiled tracheole. Each trachcole is of unicellular origin, and is at first intracellular in position; while tracheae are of multicellular origin and the lumen of each is intercellular in position. The development of tracheoles, each coiled within a single cell of the epithelium of a trachea, and the subsequent opening of communication between the tracheoles and the lumen of the trachea, and the uncoiling and stretching out of the tracheoles, so that they reach all parts of the wing.[35]
In the earlier stages of its development, the wing-bud is not provided with special organs of respiration such as tracheation, as it resembles in this respect the other portions of the hypodermis of which it is still a part. It should be noted, however, that the histoblast is developed near a large trachea, a cross-section of which is shown in, which represents sections of these parts of the first, second, third and fourth instars respectively. At the same time the tracheoles uncoil, and extend in bundles in the forming vein-cavities of the wing-bud. At the molt that marks the beginning of the pupal stadium stage, they become functional. At the same time, the larval tracheoles degenerate; their function having been replaced by the wing tracheae.[35]
Behavior
Migration
Lepidopteran migration is usually seasonal, the insects moving to escape dry seasons or other disadvantageous conditions. Most lepidopterans that migrate are butterflies, the distance travelled varying from short to very long journeys. Some butterflies that migrate include the Mourning Cloak, Painted Lady, American Lady, Red Admiral, and the Common Buckeye.[36]: 29–30 Particularly famous migrations are those of the Monarch butterfly from Mexico to northern USA and southern Canada, a distance of about 4,000–4,800 km (2,500–3,000 mi). Other well-known migratory species include the Painted Lady and several of the danaine butterflies. Spectacular and large-scale migrations associated with the Monsoons are seen in peninsular India.[37] Migrations have been studied in more recent times using wing tags and also using stable hydrogen isotopes.[38][39] Moths also undertake migrations, an example being the uraniids. Urania fulgens undergoes population explosions and massive migrations that may be not surpassed by any other insect in the Neotropics. In Costa Rica and Panama, the first population movements may begin in July and early August and, depending on the year, may be very massive, continuing unabated for as long as five months.[40]
Navigation
Navigation is important to lepidoptera species, especially for those that migrate. Butterflies, which have more species that migrate, have been shown to navigate using time compensated sun compasses. They can see polarized light and therefore can orient even in cloudy conditions. The polarized light in the region close to the ultraviolet spectrum is suggested to be particularly important.[41] It is suggested that most migratory butterflies are those that live in semi-arid areas where breeding seasons are short.[42] The life-histories of their host plants also influence the strategies of the butterflies.[43] Other theories include the use of landscapes. Lepidoptera may use coastal lines, mountains and even roads to orient themselves. Above sea it has been observed that the flight direction is much more accurate if the coast is still visible.[44]
Many studies have also shown that moths navigate. One study showed that many moths may use the Earth's magnetic field to navigate, as a study of the moth Heart and Dart suggests.[45] Another study, this time of the migratory behavior of the Silver Y, showed that even at high altitudes the species can correct its course with changing winds, and prefers flying with favourable winds, suggesting a great sense of direction.[14][46] Aphrissa statira in Panama loses its navigational capacity when exposed to a magnetic field, suggesting it uses the Earth’s magnetic field.[47]
Moths exhibit a tendency to circle artificial lights repeatedly. This suggests that they use a technique of celestial navigation called transverse orientation. By maintaining a constant angular relationship to a bright celestial light, such as the Moon, they can fly in a straight line. Celestial objects are so far away, that even after traveling great distances, the change in angle between the moth and the light source is negligible; further, the moon will always be in the upper part of the visual field or on the horizon. When a moth encounters a much closer artificial light and uses it for navigation, the angle changes noticeably after only a short distance, in addition to being often below the horizon. The moth instinctively attempts to correct by turning toward the light, causing airborne moths to come plummeting downwards, and – at close range – which results in a spiral flight path that gets closer and closer to the light source.[48] Other explanations have been suggested, such as the idea that moths may be impaired with a visual distortion called a Mach band by Henry Hsiao in 1972. He stated that they fly towards the darkest part of the sky in pursuit of safety and are thus inclined to circle ambient objects in the Mach band region.[49]
Communication
Most moths lack bright colors as many species use coloration as camouflage but butterflies engage in visual communication. Female cabbage butterflies, for example, use ultraviolet light to communicate, with scales colored in this range on the dorsal wing surface. When they fly, each down stroke of the wing creates a brief flash of ultraviolet light that the males apparently recognize as the flight signature of a potential mate. These flashes from the wings may attract several males who engage in aerial courtship displays.[50]
Males in a few groups of Lepidoptera have specially modified sets of wing scales that are associated with pheromone glands in the wings themselves, and structured in such a way as to facilitate the evaporation and dispersal of the pheromones. Perhaps the most well-known species of this type is the Monarch butterfly, in which the modified scales form a small black bulge along one of the hindwing veins.[51]
Nomenclature
Nomenclature is the system of principles, procedures and terms related to naming - which is the assigning of a word or phrase to a particular object or property, in this case assigned to an appropriate taxon.[52] Most of the of insects family nomenclature are based on the Ancient Greek name for wing, πτερόν (pteron), as the suffix -ptera.
| Scientific Name | linguistic root | Translation of the Scientific name | English Name |
|---|---|---|---|
| Anisoptera | ἀνισο- (aniso-) | Not equal | Dragonfly |
| Aptera | ἀ- (a-) meaning without | Wingless | Apterygotans, now obsolete |
| Apterygota | πτερύγιον (pterygion) (smaller) wing ἀ- (a-) meaning without |
Wingless | Apterygotans |
| Coleoptera | Κολεός (koleos, or a leather sleeve into which the sword was stuck) | Hardened wings | Beetles |
| Dermaptera | Δέρμα (derma as skin, leather) | leather wing | Earwigs |
| Diaphanopterodea | Διαφανής (diaphanes meaning transparent or translucent) | With transparent wings | diaphanopteroideans |
| Dictyoptera | Δίκτυον (diktyon, meaning network) | wings with netted venation | Cockroaches, mantids and termites |
| Diptera | Δύο- (dyo-, meaning two) | two wings | Flies |
| Embioptera | ἐ- (en as meaning inside) βίος (bios, meaning life | Interior living winged insects | webspinners |
| Endopterygota | ἐντός (entos as meaning inside) πτερύγιον or (smaller) wing | Inner wing | Insects of holometabola |
| Ephemeroptera | ἐφήμερος (ephemeros about one day long) | Day flying | Mayflies |
| Exopterygota | ἔξω (exo, or external) | Outdoor flying insects | Insects that undergo incomplete metamorphasis |
| Hemiptera | ἡμι- (hemi-, or half, semi-) | halfwinged insects | Hemiptera (bugs, leafhoppers, aphids, etc.) |
| Heteroptera | ἑτερο- (hetero- or different) | Different winged | Bugs |
| Homoptera | ὅμο- (homo- or same, similar) | Same winged | Leafhoppers, aphids, etc. |
| Hymenoptera | ὑμένιον (hymenion or -thin- membrane) | insects with wings with thin membranes | bees, ants, etc. |
| Isoptera | ἶσον (ison meaning equal) | Same winged | Termites |
| Lepidoptera | Λεπίς (lepis meaning scales) | Scaled wings | Butterflies & Moths |
| Lonchopteridae | Λόγχη (lonchi, meaning lance or spear) | Lance wing | Lance flies |
| Mecoptera | μῆκος (mekos or length) | Long wing | Snake flies, etc. |
| Megaloptera | Μεγαλο- (megalo- or simple large) | large wings | Mud flies |
| Neuroptera | νεῦρον (neuron meaning nerve, or vein) | Veined wing | Lacewings |
| Neoptera | νέος (neos new / young) | New wing | Includes all currently living orders of flying insects out of mayflies and dragonflies |
| Oligoneoptera | ὀλίγον- (oligon- meaning little) νέος (neos or new) |
New with little veins | Division of the Neoptera |
| Orthoptera | ὀρθο (ortho- meaning straight, correct) | straight wing | Grasshoppers and crickets |
| Palaeodictyoptera | Παλαιός (palaios- meaning old) δίκτυον (diktyon meaning network) |
old veined wings | primitive palaeozoic paleopterous insects |
| Palaeoptera | Παλαιόν (Palaion meaning old) | Old wings | Mayflies, etc. |
| Paraneoptera | Παρα- (Para-) νέος (neos meaning new) |
Neoptera with fake wings (Hemielytren, Paraelytren ...) | Bugs, earwigs ... |
| Phthiraptera | Φθείρ (phtheir meaning lice) ἀ, a- meaning without |
Lice without wings | Animal lice |
| Plecoptera | Πλέκειν (plekein meaning fold) | folded wings | Stoneflies |
| Polyneoptera | Πολύς (polys, meaning a lot of ) νέοςneosnew |
Many veined wings | Neopterans undergoing incomplete metamorphosis |
| Psocoptera | Ψώχω (psocho meaning to rub) | Rubing wings | Booklice |
| Pterygota | Πτερύγιον pterygion'(small) wing | Wing insects | In class, unlike Apterygota, including winged and wingless secondary systems |
| Raphidioptera | ῥαφίς (rhaphis meaning needle) | Needle wing | Snakeflies |
| Siphonaptera | Σίφων (siphon meaning suction or tube) ἀ- or without |
Wingless siphon | Fleas |
| Strepsiptera | Στρέψις (strepsis meaning to turn around) | Rotating or twisted wing | twisted-winged parasites |
| Thysanoptera | Θύσανοι (thysanoi meaning fringe) | Fring winged | Thrips |
| Trichoptera | Τρίχωμα (trichoma meaning hair) | Haired wing | Caddisflies |
| Zoraptera | Ζωρός (zōros meaning strong) | Strong wing | zorapterans |
| Zygoptera | ζεῦγος (zeugos meanign pair) | Paired wings | Damselflies |
Adaptations
Variation
Insect wings are fundamental in identifying and classifying species as there is no other set of structures in studying insects more significant. Each order and insect family has distinctive wing shapes and features. In many cases, even species may be distinguished from each other by differences of color and pattern. For example, just by position one can identify species, albeit to a much lesser extent. Though most insects fold their wings when at rest, dragonflies and some damselflies rest with their wings spread out horizontally, while groups such as the caddisflies, stoneflies, alderflies, and lacewings hold their wings sloped roof-like over their backs. A few moths wrap their wings around their bodies, while many flies and most butterflies close their wings together straight upward over the back.[20]
Many times the shape of the wings correlates with the type of insect flight. The best flying insects tend to have long, slender wings. In many species of Sphingidae (sphinx moths), the forewings are large and sharply pointed, forming with the small hind wings a triangle that is suggestive of the wings of fast, modern airplanes. Another, possibly more important correlation, is that of the size and power of the muscles to the speed and power of flight. In the powerfully flying insects, the wings are most adapted for the stresses and aerodynamics of flight. The veins are thicker, stronger, and closer together toward the front edge (or "leading edge") and thinner yet flexible toward the rear edge (or "trailing edge"). This makes the insect wing an excellently constructed airfoil, capable of exerting both propulsion and lift while minimizing drag.[20]
Variation of the wing beat may also occur, not just amongst different species, but even among individuals at different times. In general, the frequency is dependent upon the ratio between the power of the wing muscles and the resistance of the load. Large-winged, light-bodied butterflies may have a wing beat frequency of 4–20 per second whereas small-winged, heavy-bodied flies and bees beat their wings more than 100 times a second and mosquitoes can beat up to 988–1046 times a second. The same goes for flight; though it is generally difficult to estimate the speed of insects in flight, most insects can probably fly faster in nature than they do in controlled experiments.[20]
Coleoptera
In species of Coleoptera (beetles), the only functional wings are the hind wings. The hind wing is longer than the elytra, folded longitudinally and transversely under the elytra. The wing is rotated forwards on its base into flight position. This action spread the wing and unfolded longitudinally and transversely. There is the spring mechanism in the wing structure, sometimes with the help of abdomen movement, to keep the wing in folded position. The beetle wing venation is reduced and modified due to the folding structure, which include:[53]
- Costa (C), Subcosta posterior (ScP) - at the leading wing marginal, fused for most of the length.
- Radius anterior (RA) - divided into two branches beyond the middle of the wing.
- Radius posterior (RP) - basal connection is lost.
- Media posterior (MP) - branches, long and strong vein.
- Cubitus anterior (CuA)
- Anal veins (AA, AP) - veins behind the cubitus, separated by anal fold.
In most species of beetles, the front pair of wings are modified and sclerotised (hardened) to form elytra and they protect the delicate hindwings which are folded beneath.[23] The elytra is connected to the pterathorax; being called as such because it is where the wings are connected (pteron meaning "wing" in Greek). The elytra are not used for flight, but tend to cover the hind part of the body and protect the second pair of wings (alae). The elytra must be raised in order to move the hind flight wings. A beetle's flight wings are crossed with veins and are folded after landing, often along these veins, and are stored below the elytra. In some beetles, the ability to fly has been lost. These include some ground beetles (family Carabidae) and some "true weevils" (family Curculionidae), but also some desert and cave-dwelling species of other families. Many of these species have the two elytra fused together, forming a solid shield over the abdomen. In a few families, both the ability to fly and the elytra have been lost, with the best known example being the glow-worms of the family Phengodidae, in which the females are larviform throughout their lives.[3][23]
Lepidoptera
The two pairs of wings are found on the middle and third segment, or mesothorax and metathorax respectively. In the more recent genera, the wings of the second segment or much more pronounced, however some, more primitive form, have similarly sized wings of both segments. The wings are covered in scales arranged like shingles, forming the extraordinary variety seen in color. The mesothorax is designed to have more powerful muscles to propel moth or butterfly through the air, with the wing of said segment having a stronger vein structure.[23]: 560 The largest superfamily, Noctuidae, has the wings modified to act as Tympanal or hearing organs[54] Modifications in the wing's venation include:[53]
- Costa (C) - not found in Butterflies.
- Subcosta (Sc) + Radius 1 (Sc+R1) - at the leading wing marginal, fused or very close for most of the length, in hind wing fused and well developed in the humeral area, subcosta never branches in butterfly.
- Radius (R2-R5) - radius divides into branches beyond the middle of the wing up to five branches in Papilionidae. On forewing, the last R is stalked in all butterflies except Hesperiidae is separated.
- Radius sector (Rs) - in hind wing.
- Media (M1-M3) - the basal section has been lost.
- Cubitus anterior (CuA1-CuA2) - CuP section has been lost.
- Anal veins (A, 1A+2A, 3A) - either one vein A, or two veins 1A+2A, 3A.
- Humeral vein - The hind wing of most butterflies has the humeral vein, except Lycaenidae There is the enlargement of the humeral area of the hind wing which is overlapped with the fore wing. The humeral vein strengthened the hind wing overlapped area so that the two wings coupling better.
The wings, head parts of thorax and abdomen of Lepidoptera are covered with minute scales, from which feature the order 'Lepidoptera' derives its names, the word "lepteron" in Ancient Greek meaning 'scale'. Most scales are lamellar, or blade-like and attached with a pedicel, while other forms may be hair-like or specialized as secondary sexual characteristics.[55] The lumen or surface of the lamella, has a complex structure. It gives color either due to the pigmentary colors contained within or due to its three-dimensional structure.[56] Scales provide a number of functions, which include insulation, thermoregulation, aiding gliding flight, amongst others, the most important of which is the large diversity of vivid or indistinct patterns they provide which help the organism protect itself by camouflage, mimicry, and to seek mates.[55]
Odonata
Species of Odonata (Damselflies and dragonflies) both have two pairs of wings which are about equal in size and shape and are clear in color. There are five, if the R+M is counted as 1, main vein stems on dragonfly and damselfly wings, and wing veins are fused at their bases and the wings cannot be folded over the body at rest, which also include:[53]
- Costa (C) -- at the leading edge of the wing, strong and marginal, extends to the apex of the wing.
- Subcosta (Sc) -- second longitudinal vein, it is unbranched, joins C at nodus.
- Radius and Media (R+M) -- third and fourth longitudinal vein, the strongest vein on the wing, with branches, R1-R4, reach the wing margin, the media anterior (MA) are also reach the wing margin. IR2 and IR3 are intercalary veins behind R2 and R3 respectively.
- Cubitus (Cu) -- fifth longitudinal vein, cubitus posterior (CuP) is unbranched and reach the wing margin.
- Anal veins (A1) -- unbranched veins behind the cubitus.
- A nodus is formed where the second main vein meets the leading edge of the wing. The black pterostigma is carried near the wing tip.
The main veins and the crossveins form the wing venation pattern. The venation patterns are different in different species. There may be very numerous crossveins or rather few. The Australian Flatwing Damselfly's wings are one of the few veins patterns. The venation pattern is useful for species identification.[53] Almost all Anisoptera settle with the wings held out sideways or slightly downward, however most settle with the wings held together, dorsal surfaces apposed. The thorax of Zygoptera is so oblique that when held in this way the wings fit neatly along the top of the abdomen. They do not appear to be held straight up as in butterflies or mayflies. In a few zygopteran families the wings are held horizontally at rest, and in one anisopteran genus (e.g. Cordulephya, Corduliidae) the wings are held in the typical damselfly resting position. Adult species possess two pairs of equal or subequal wings. There appear to be only five main vein stems. A nodus is formed where the second main vein (subcosta) meets the leading edge of the wing. In most families a conspicuous pterostigma is carried near the wing tip. Identification as Odonata can be based on the venation. The only likely confusion is with some lacewings (order Neuroptera) which have many crossveins in the wings. Until the early years of the 20th century Odonata were often regarded as being related to lacewings and were given the ordinal name Paraneuroptera, but any resemblance between these two orders is entirely superficial. In Anisoptera the hindwing is broader than the forewing and in both wings a crossvein divides the discoidal cell into a Triangle and Supertriangle.[57]
Orthoptera

Species of Orthoptera (Grasshoppers and crickets) have forewings that are tough opaque tegmina, narrow which are normally covering the hind wings and abdomen at rest. The hind wings are board membranous and folded in fan-like manner, which include the following venation:[53]
- Costa (C) - at the leading marginal of the forewing and hind wing, unbranched.
- Subcosta (Sc) - second longitudinal vein, unbranched.
- Radius (R) - third longitudinal vein, branched to Rs in forewing and hind wing.
- Media anterior (MA) - fourth longitudinal vein, branched in basal part as Media posterior (MP).
- Cubitus (Cu) - fifth longitudinal vein, on forewing and hind wing dividing near the wing base into branched CuA, and unbranched CuP.
- Anal veins (A) - veins behind the cubitus, unbranched, two in forewing, many in hind wing.
Phasmatodea

- Costa (C) - at the leading marginal of the hind wing, unbranched, absent in forewing.
- Subcosta (Sc) - second longitudinal vein, unbranched.
- Radius (R) - third longitudinal vein, branched to Rs in hind wing, unbranched in forewing.
- Media anterior (MA) - fourth longitudinal vein, branched in basal part as Media posterior (MP).
- Cubitus (Cu) - fifth longitudinal vein, unbranched.
- Anal veins (A) - veins behind the cubitus, unbranched, two in forewing, many in hind wing 1A-7A in one group and the rest in another group.
Stick insect have forewings that are tough, opaque tegmina, short and covering only the base part of the hind wings at rest. Hind wings from costa to Cubitus are tough and opaque like the forewings. The large anal area are membranous and folded in fan-like manner. There are no or very few branching in Stick Insect wing veins.[53]
Dermaptera
Other orders such as the Dermaptera (earwigs), Orthoptera (grasshoppers, crickets), Mantodea (praying mantis) and Blattodea (cockroaches) have rigid leathery forewings that aren't used for flying, sometimes called tegmen (pl. tegmina), elytra, or pseudoelytron.[3]
Hemiptera
In Hemiptera (true bugs), the forewings may be hardened, though to a lesser extent than in the beetles. For example, the anterior part of the front wings of stink bugs is hardened, while the posterior part is membranous. They are called hemelytron (pl. hemelytra). They are only found in the suborder Heteroptera; the wings of the Homoptera, such as the cicada, are typically entirely membranous. Both forewings and hindwings of Cicada are membranous, most species are glass-like although some are opaque. Cicadas are not good fliers and most fly only a few seconds. When fly, forewing and hind wing are hooked together by a grooved coupling along the hind wing costa and forewing margin. Most species have a basic venation as shown in the following picture.[53]

- Costa (C) - at the leading wing marginal, in forewing extends to the node and lies close to Sc+R.
- Subcosta + Radius (Sc+R) - in forewing Sc and R fused together to the node. Radial sector (Rs) arises near the node and unbranches.
- Radius anterior (RA)
- Radius posterior (RP)
- Media (M) - branches to M1 to M4.
- Cubitus anterior (CuA) - branches to CuA1 and CuA2.
- Cubitus posterior (CuP) - unbranches.
- Anal veins (A) - veins behind the cubitus, 1A and 2A fused in the forewing, CuP and 2A are folded.
Also notice there are the ambient veins and peripheral membranes on the margin of both wings.
Diptera
In the Diptera (true flies), there are only one pair of functional wings, with the posterior pair of wings are reduced to halteres, which help the fly to sense its orientation and movement, as well as to improve balance by acting similar to gyroscopes. In Calyptratae, the very hindmost portion of the wings are modified into somewhat thickened flaps called calypters which cover the halteres.[53]

- Costa (C) - not found in Diptera.
- Subcosta (Sc) - became the leading wing vein, unbranched.
- Radius (R) - branched to R1-R5.
- Media (M) - branched to M1-M4.
- Cubitus anterior(CuA)- unbranched, CuP is reduced in Diptera. Some species CuA and 1A are separated, some species meets when reaching the wing margin, some species fused.
- Anal veins (A) - only two anal veins 1A and 2A are present, 2A is not distinctive in some species.
- Discal Cell (dc) - well define in most species.
Blattodea
Species of Blattodea (cockroaches) have a forewing, are also known as tegmen, that is more or less sclerotized. It is used in flight as well as a form of protection of the membranous hind wings. The veins of hind wing are about the same as front wing but with large anal lobe folded at rest between CuP and 1A. The anal lobe usually folded in a fan-like manner.[53]

- Costa (C) - at the leading edge of the wing.
- Subcosta (Sc) - second longitudinal vein, it is relatively short.
- Radius (R) - third longitudinal vein, with many pectinate branches.
- Media (M) - fourth longitudinal vein, reach the wing margin.
- Cubitus anterior (CuA) - fifth longitudinal vein, with dichotomous branches occupy large part of tegmen.
- Cubitus posterior (CuP) - is unbranched, curved and reach the wing margin.
- Anal veins (A) - veins behind the cubitus.
Hymenoptera
| An example of Longitudinal folding in wasps (Vespidae) | |

|
The main fold line of the forewing seen half way up as a bright horizontal line. The wing part that is behind this line is turned back down. The narrow strip at the front edge of the wing is in front of the first strong wire folded forward and down. |

|
So in rest position, the outer lining forms the tough outer edge of the wing, which protects the sides of the abdomen as a shock absorber. The rear wing is covered largely by the forewing. |
The Hymenoptera adults, include sawflies, wasps, bees and non-working ants, all of which have two pairs of membranous wings.[53]
- Costa (C) - not found in Hymenoptera.
- Subcosta (Sc) - unbranched.
- Radius (R) - branched to R1-R5.
- Media (M) - M is unbranched, in forewing M is fused with Rs for part of its length.
- Cubitus (CuA) - unbranched, CuP is absent in Hymenoptera.
- Anal veins (A) - only two anal veins 1A and 2A are present, 2A is not distinctive in some species.
- Wing-coupling - Row of hooks on the leading edge of hind wing engage the hind margin of the forewing, strongly couple the wings in flight.
- Line of wing folding - Some species, including Vespidae, the forewing are longitudinally folded along the 'line of wing folding' at rest.
- Pterostigma - is present for some species.
The forward margin of the hind wing bears a number of hooked bristles, or "hamuli", which lock onto the fore wing, keeping them held together. The smaller species may have only two or three hamuli on each side, but the largest wasps may have a considerable number, keeping the wings gripped together especially tightly. Hymenopteran wings have relatively few veins compared with many other insects, especially in the smaller species.[3]
Other families
Termites are relatively poor fliers and are readily blown downwind in wind speeds of less than 2 km/h, shedding their wings soon after landing at an acceptable site, where they mate and attempt to form a nest in damp timber or earth.[58] Wings of most termites have three heavy veins along the basal part of the front edge of the forewing and the crossveins near the wing tip are angled, making trapezoidal cells. Although subterranean termite wings have just two major veins along the front edge of the forewing and the cross veins towards the wingtip are perpendicular to these veins, making square and rectangular cells.[59]
Species of Thysanoptera (thrips) have slender front and hind wings with long fringes of hair, called fringed wings. While species of Trichoptera (caddisfly) have hairy wings with the front and hind wings clothed with setae.[3]
See also
Notes
- ^ a b c d e f Chapman, R.F. (1998). The Insects: Structure and function (4th ed.). Cambridge, New York: Cambridge University Press. ISBN 0-521-57048-4.
- ^ a b Gilliott, Cedric (August 1995). Entomology (2 ed.). Springer-Verlag New York, LLC. ISBN 0-306-44967-6.
- ^ a b c d e Meyer, John R. (5 January 2007). "External Anatomy: WINGS". Department of Entomology, NC State University. Retrieved 2011-03-21.
- ^ a b c d Gullan, P. J. (2004). The insects: an outline of entomology. UK: Blackwell Publishing. ISBN 1-4051-1113-5.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k l m n o Snodgrass, R. E. (December 1993). Principles of Insect Morphology. Cornell Univ Press. ISBN 0-8014-8125-2.
- ^ Spieth, HT (1932). "A New Method of Studying the Wing Veins of the Mayflies and Some Results Therefrom (Ephemerida)" (PDF). Entomological News.
- ^ a b c Knospe, Carl R. (Fall 1998). "Insect Flight Mechanisms: Anatomy and Kinematics" (PDF). Mechanical and Aerospace Engineering, University of Virginia.
- ^ "DIFFUSION IN INSECT WJNG MUSCLE,THE MOST ACTIVE TISSUE KNOWN". http://jeb.biologists.org/content/41/2/229.full.pdf+html. 41: 229–256. July 1963.
{{cite journal}}: External link in|journal= - ^ Tiegs, O. W. (February 1954). "The Flight Muscles of Insects-Their Anatomy and Histology; with Some Observations on the Structure of Striated Muscle in General". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 238 (656): 221–348.
- ^ I.P. Woiwod, D.R. Reynolds and C.D. Thomas(Eds) 2001. Insect Movement: Mechanisms and Consequences. CAB International.
- ^ Joos, B. (1987). "Carbohydrate use in the flight muscles of Manduca sexta during pre-flight warm-up". Journal of Experimental Biology. 133: 317–327.
- ^ "Definition of Synchronous muscle in the Entomologists' glossary". Department of Entomology, NC State University. Retrieved 2011-03-21.
- ^ "Definition of Asynchronous muscle in the Entomologists' glossary". Department of Entomology, NC State University. Retrieved 2011-03-21.
- ^ a b c d e Chapman, A. D. (2006). Numbers of living species in Australia and the World. Canberra: Australian Biological Resources Study. pp. 60pp. ISBN 978-0-642-56850-2. Cite error: The named reference "Chapman" was defined multiple times with different content (see the help page).
- ^ Smith D.S. (1965). "Flight muscles of insects". Scientific American. 212 (6): 76–88. doi:10.1038/scientificamerican0665-76.
- ^ a b c d e f g h i j k Wang, Z. Jane (2005). "DISSECTING INSECT FLIGHT" (pdf). Annual Reviews. Bibcode:2005AnRFM..37..183W. doi:10.1146/annurev.fluid.36.050802.121940.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e f g Sane, Sanjay P. (2003). "The aerodynamics of insect flight" (PDF). The Journal of Experimental Biology. 206: 4191–4208. doi:10.1242/jeb.00663. PMID 14581590.
- ^ a b c d e f g h i j k l m n o p q r s t u v Davidovits, Paul (2008). Physics in Biology and Medicine. Academic Press. pp. 78–79. ISBN 978-0-12-369411-9.
- ^ "Catching the Wake". Scientific American. June 28, 1999. Retrieved March 31, 2011.
- ^ a b c d e "Insect Wings in General". Aerodynamics of Insects. Cislunar Aerospace. 1997. Retrieved March 28, 2011.
- ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1098/rspb.2010.0170, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1098/rspb.2010.0170instead. - ^ a b c d e f g h i Grimaldi, David (2005). Evolution of the Insects. New York, NY: Cambridge University Press.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e Powell, Jerry A. (2009). "Coleoptera". In Resh, Vincent H.; Cardé, Ring T. (eds.). Encyclopedia of Insects (2 (illustrated) ed.). Academic Press. p. 1132. ISBN 978-0-12-374144-8. Retrieved 14 November 2010.
{{cite book}}: Cite has empty unknown parameter:|chapterurl=(help) - ^ Michael S. Engel & David A. Grimaldi (2004). "New light shed on the oldest insect". Nature. 427 (6975): 627–630. doi:10.1038/nature02291. PMID 14961119.
- ^ Grzimek HC Bernhard (1975) Grzimek's Animal Life Encyclopedia Vol 22 Insects. Van Nostrand Reinhold Co. NY.
- ^ Riek EF Kukalova-Peck J (1984) A new interpretation of dragonfly wing venation based on early Upper Carboniferous fossils from Argentina (Insecta: Odonatoida and basic character states in Pterygote wings.) Can. J. Zool. 62; 1150-1160.
- ^ Wakeling JM Ellington CP (1997) Dragonfly flight III lift and power requirements. Journal of Experimental Biology 200; 583-600, on p589
- ^ Matsuda R (1970) Morphology and evolution of the insect thorax. Mem. Ent. Soc. Can. 76; 1-431.
- ^ Riek EF Kukalova-Peck J (1984) A new interpretation of dragonfly wing venation based on early Upper Carboniferous fossils from Argentina (Insecta: Odonatoida and basic character states in Pterygote wings.) Can. J. Zool. 62; 1150-1160
- ^ V. A. Blagoderov, E. D. Lukashevich & M. B. Mostovski (2002). "Order Diptera Linné, 1758. The true flies". In A. P. Rasnitsyn & D. L. J. Quicke (ed.). History of Insects. Kluwer Academic Publishers. ISBN 1-4020-0026-X.
- ^ Trueman JWH (1990), Comment: evolution of insect wings: a limb exite plus endite model Canadian Journal of Zoology
- ^ Staniczek, A.H. & Bechly, G. & Godunko, R.J. (2011): Coxoplectoptera, a new fossil order of Palaeoptera (Arthropoda: Insecta), with comments on the phylogeny of the stem group of mayflies (Ephemeroptera). Insect Systematics & Evolution, 42(2): 101-138, Brill, Leiden. ISSN 1399-560X PDF fulltext
- ^ Adrian L. R. Thomas & R. Åke Norberg (1996) Skimming the surface — the origin of flight in insects? Trends in Ecology & Evolution 11(5):187-188 doi:10.1016/0169-5347(96)30022-0
- ^ Yanoviak SP, Kaspari M, Dudley R. (2009). Gliding hexapods and the origins of insect aerial behaviour. Biol Lett. 5(4):510-2. doi:10.1098/rsbl.2009.0029 PMID 19324632
- ^ a b c d H. Comstock, Henry (1918). The Wings of Insects. Ithaca, NY: The Comstack Publishing Company.
- ^ Dole, Claire Hagen (May 28, 2003). The Butterfly Gardener's Guide. Brooklyn Botanic Garden. ISBN 1-889538-58-2.
- ^ Williams, C. B. (1927). "A study of butterfly migration in south India and Ceylon, based largely on records by Messrs. G. Evershed, E. E. Green, J. C. F. Fryer and W. Ormiston". Transactions of the Royal Entomological Society of London. 75 (1): 1–33. doi:10.1111/j.1365-2311.1927.tb00054.x.
- ^ Urquhart, F. A. & N. R. Urquhart. 1977. Overwintering areas and migratory routes of the Monarch butterfly (Danaus p. plexippus, Lepidoptera: Danaidae) in North America, with special reference to the western population. Can. Ent. 109: 1583–1589
- ^ Wassenaar L. I. & K. A. Hobson (1998). "Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence". Proceedings of the National Academy of Sciences. 95 (26): 15436–15439. Bibcode:1998PNAS...9515436W. doi:10.1073/pnas.95.26.15436. PMC 28060. PMID 9860986.
- ^ Smith, N. G. (1983). Urania fulgens (Calipato Verde, Green Urania). Costa Rican Natural History. Chicago: University of Chicago Press. p. 816.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sauman, Ivo (May 5, 2005). "Connecting the Navigational Clock to Sun Compass Input in Monarch Butterfly Brain". Neuron. 46 (3): 457–467. doi:10.1016/j.neuron.2005.03.014. PMID 15882645.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Southwood, T. R. E. (1962). "Migration of terrestrial arthropods in relation to habitat". Biological Reviews. 37 (2). Cambridge Philosophical Society: 171–211. doi:10.1111/j.1469-185X.1962.tb01609.x.
- ^ Dennis, Roger L. H. (September 2005). "Does diet breadth control herbivorous insect distribution size? Life history and resource outlets for specialist butterflies" (PDF). Journal of Insect Conservation. 9 (3). Springer Netherlands: 187–200. doi:10.1007/s10841-005-5660-x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Made, J. G. van der (1989). Actie voor Vlinders, zo kunnen we ze redden (in Dutch). Weert: M & P cop. p. 192. ISBN 90-6590-303-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Baker, R. Robin (February 1987). google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=2892ea838fdde22df54095b4505c1c2b "Integrated use of moon and magnetic compasses by the heart-and-dart moth, Agrotis exclamationis". Animal Behaviour. 35 (1): 94–101. doi:10.1016/S0003-3472(87)80214-2.
{{cite journal}}: Check|url=value (help) - ^ Breen, Amanda (May 7, 2008). "Scientists make compass discovery in migrating moths". University of Greenwich at Medway. p. 1. Retrieved December 9, 2009.
- ^ Srygley, Robert B. (2005). "Experimental evidence for a magnetic sense in Neotropical migrating butterflies (Lepidoptera: Pieridae)". The British Journal of Animal Behaviour. 71 (1): 183–191. doi:10.1016/j.anbehav.2005.04.013. ISSN 0003-3472.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Elliot, Debbie (August 18, 2007). "Why are Moths Attracted to Flame? (audio)". National Public Radio. p. 1. Retrieved December 12, 2009.
{{cite news}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hsiao, Henry S. (1972). Attraction of moths to light and to infrared radiation. San Francisco Press. ISBN 0-911302-21-2.
- ^ Meyer, John R. (2006). "Acoustic Communication". Department of Entomology, C State University. Retrieved 25 February 2011.
- ^ Hall, Jason P. W.; Harvey, Donald J. (2002). "A survey of androconial organs in the Riodinidae (Lepidoptera)" (PDF). Zoological Journal of the Linnean Society. 136 (2): 171–197. doi:10.1046/j.1096-3642.2002.00003.x.
- ^ "Nomenclature - definitions from Dictionary.com". Retrieved 2007-10-06.
- ^ a b c d e f g h i j Chew, Peter (May 9, 2009). "Insect Wings". Brisbane Insects and Spiders. Retrieved 2011-03-21.
- ^ Scoble, MJ. (1992). The Lepidoptera: Form, function, and diversity. Oxford Univ. Press. ISBN 978-1-4020-6242-1.
- ^ a b Scoble (1995). Section Scales, (pp 63 - 66).
- ^ Vukusic, P. (2006). "Structural color in Lepidoptera" (PDF). Current Biology. 16 (16): R621–3. doi:10.1016/j.cub.2006.07.040. PMID 16920604. Retrieved 11 November 2010.
- ^ Trueman, John W. H. (16 October 2009). "Odonata. Dragonflies and damselflies". Tolweb.org. Retrieved 2011-03-21.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Abe T., Bignell D.E (2000). Termites: evolution, sociality, symbioses, ecology, ecolab. Kluwer academic publishers. ISBN 0-7923-6361-2.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "Termite". Texas AgriLife Extension Service.
References
- Triplehorn, Charles A. (2005). Borror and DeLong's introduction to the study of insects (7th edition ed.). Thomson Brooks/Cole. ISBN 0-03-096835-6.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- Brisbane University course on insect wings
- North-Carolina state University course on insect wings
- Insect wing drawings


























