Cantharidin: Difference between revisions
m Dating maintenance tags: {{Missing information}} |
Minor grammar fixes & syntax revisions. Matched verb tenses. |
||
| Line 90: | Line 90: | ||
}} |
}} |
||
'''Cantharidin''' is an odorless, colorless fatty substance of the [[terpenoid]] class, which is secreted by many species of [[blister beetle]]s.{{efn|Including broadly in genus ''[[Epicauta]]'', genus ''[[Berberomeloe]]'', and in species [[Lytta vesicatoria|''Lytta vesicatoria'' (Spanish fly)]]. [[Oedemeridae|False blister beetles]], [[Pyrochroidae|cardinal beetles]], and [[soldier beetles]] also produce cantharidin.}} It is a burn agent or a poison in large doses, but preparations containing it were historically used as [[aphrodisiac]]s ([[Spanish fly]]). In its natural form, cantharidin is secreted by the male blister beetle and given to the female as a copulatory gift during mating. Afterwards, the female beetle covers her eggs with it as a defense against predators. |
'''Cantharidin''' is an odorless, colorless fatty substance of the [[terpenoid]] class, which is secreted by many species of [[blister beetle]]s.{{efn|Including broadly in genus ''[[Epicauta]]'', genus ''[[Berberomeloe]]'', and in species [[Lytta vesicatoria|''Lytta vesicatoria'' (Spanish fly)]]. [[Oedemeridae|False blister beetles]], [[Pyrochroidae|cardinal beetles]], and [[soldier beetles]] also produce cantharidin.}} It is a burn agent or a [[poison]] in large doses, but preparations containing it were historically used as [[aphrodisiac]]s ([[Spanish fly]]). In its natural form, cantharidin is secreted by the male blister beetle, and given to the female as a copulatory gift during mating. Afterwards, the female beetle covers her eggs with it as a defense against predators. |
||
Poisoning from cantharidin is a significant veterinary concern, especially in horses, but it can also be poisonous to humans if taken internally (where the source is usually experimental self-exposure). Externally, cantharidin is a potent [[vesicant]] (blistering agent), exposure to which can cause severe [[chemical burn]]s. Properly dosed and applied, the same properties have also been used therapeutically, for instance for treatment of skin conditions such as [[molluscum contagiosum]] infection of the skin. |
Poisoning from cantharidin is a significant veterinary concern, especially in horses, but it can also be poisonous to humans if taken internally (where the source is usually experimental self-exposure). Externally, cantharidin is a potent [[vesicant]] (blistering agent), exposure to which can cause severe [[chemical burn]]s. Properly dosed and applied, the same properties have also been used therapeutically, for instance, for treatment of skin conditions, such as [[molluscum contagiosum]] infection of the skin. |
||
Cantharidin is classified as an [[List of extremely hazardous substances|extremely hazardous substance]] in the United States and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities.<ref>As defined in Section 302 of the U.S. [[Emergency Planning and Community Right-to-Know Act]] (42 U.S.C. 11002). See [http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities"] {{Webarchive|url=https://web.archive.org/web/20120225051612/http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf |date=2012-02-25 }} (PDF) (July 1, 2008 ed.). U.S. Government Printing Office. Retrieved October 29, 2011.</ref> |
Cantharidin is classified as an [[List of extremely hazardous substances|extremely hazardous substance]] in the [[United States]], and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities.<ref>As defined in Section 302 of the U.S. [[Emergency Planning and Community Right-to-Know Act]] (42 U.S.C. 11002). See [http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities"] {{Webarchive|url=https://web.archive.org/web/20120225051612/http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf |date=2012-02-25 }} (PDF) (July 1, 2008 ed.). U.S. Government Printing Office. Retrieved October 29, 2011.</ref> |
||
==Chemistry== |
==Chemistry== |
||
| Line 101: | Line 101: | ||
Cantharidin, from the Greek ''kantharis'', for beetle,<ref>{{cite book | title = A Dictionary of Entomology | date = 2011 | publisher = CABI | page = 253}}</ref> is an odorless, colorless [[natural product]] with solubility in various organic solvents,{{specify|date=December 2015}} but only slight solubility in water.<ref name = veterinary>{{cite book | author = Schmitz, David G. | editor = Aiello, Susan E.| editor2 = Moses, Michael A. | year = 2013 | title = The Merck Veterinary Manual | chapter = Overview of Cantharidin Poisoning (Blister Beetle Poisoning) | location = Kenilworth, NJ, USA | publisher = Merck Sharp & Dohme | isbn = 978-0911910612 | chapter-url = https://www.merckvetmanual.com/mvm/toxicology/cantharidin_poisoning/overview_of_cantharidin_poisoning.html | access-date = 14 December 2015 }}</ref> Its skeleton is [[tricyclic]], formally, a tricyclo-[5.2.1.0<sup>2,6</sup>]decane skeleton. Its functionalities include a [[Organic acid anhydride|carboxylic acid anhydride]] (−CO−O−CO−) substructure in one of its rings, as well as a bridging [[ether]] in its [[bicyclic]] ring system. |
Cantharidin, from the Greek ''kantharis'', for beetle,<ref>{{cite book | title = A Dictionary of Entomology | date = 2011 | publisher = CABI | page = 253}}</ref> is an odorless, colorless [[natural product]] with solubility in various organic solvents,{{specify|date=December 2015}} but only slight solubility in water.<ref name = veterinary>{{cite book | author = Schmitz, David G. | editor = Aiello, Susan E.| editor2 = Moses, Michael A. | year = 2013 | title = The Merck Veterinary Manual | chapter = Overview of Cantharidin Poisoning (Blister Beetle Poisoning) | location = Kenilworth, NJ, USA | publisher = Merck Sharp & Dohme | isbn = 978-0911910612 | chapter-url = https://www.merckvetmanual.com/mvm/toxicology/cantharidin_poisoning/overview_of_cantharidin_poisoning.html | access-date = 14 December 2015 }}</ref> Its skeleton is [[tricyclic]], formally, a tricyclo-[5.2.1.0<sup>2,6</sup>]decane skeleton. Its functionalities include a [[Organic acid anhydride|carboxylic acid anhydride]] (−CO−O−CO−) substructure in one of its rings, as well as a bridging [[ether]] in its [[bicyclic]] ring system. |
||
[[File:Cantharidin biosynthesis2.png|thumb|left|Biosynthesis from farnesol — bonds to be formed and major atoms to be added are in {{color|blue|blue}} while bonds to be broken and atoms/structural segments to be removed are in {{color|red|red}}]] |
[[File:Cantharidin biosynthesis2.png|thumb|left|Biosynthesis from farnesol — bonds to be formed and major atoms to be added are in {{color|blue|blue}}; while bonds to be broken and atoms/structural segments to be removed are in {{color|red|red}}.]] |
||
The complete mechanism of the [[biosynthesis]] of cantharidin is unknown. Its framework formally consists of two [[isoprene]] units.<ref>{{Cite book | title = Secondary-Metabolite Biosynthesis and Metabolism | author = Richard J. Petroski, Susan P. McCormick | publisher = Springer Science & Business Media | date = 2012}}</ref> |
The complete mechanism of the [[biosynthesis]] of cantharidin is unknown. Its framework formally consists of two [[isoprene]] units.<ref>{{Cite book | title = Secondary-Metabolite Biosynthesis and Metabolism | author = Richard J. Petroski, Susan P. McCormick | publisher = Springer Science & Business Media | date = 2012}}</ref> However, [[feeding studies]] indicate that the biosynthetic process is more complicated, and not a simple product of [[geranyl pyrophosphate]] or related ten-carbon parent structure, as the seeming [[monoterpene]] nature would suggest. Instead, there is a [[farnesol]] (15-carbon) precursor from which certain carbon segments are later excised.<ref>{{cite journal |journal= J Insect Sci |year= 2017 |volume= 17 |issue= 2 |pages= 52 |doi= 10.1093/jisesa/iex021 |pmc= 5633858 |pmid= 28423415 |title= The Potential Organ Involved in Cantharidin Biosynthesis in ''Epicauta chinensis'' Laporte (Coleoptera: Meloidae) |first1= Ming |last1= Jiang |first2= Shumin |last2= Lü |first3= Yalin |last3= Zhang }}</ref> |
||
===Distribution and availability=== |
===Distribution and availability=== |
||
| Line 113: | Line 113: | ||
===Aphrodisiac preparations=== |
===Aphrodisiac preparations=== |
||
Preparations made from [[Spanish fly|blistering beetles]] have been used since ancient times as an [[aphrodisiac]], possibly because their physical effects were perceived to mimic those of sexual arousal,<ref>John L. Capinera, ''Encyclopedia of Entomology, Volume 4'', Springer Science & Business Media, 2008. p.2010</ref> and because they can cause prolonged erection or [[priapism]] in men.<ref name = aph>Peter V. Taberner, ''Aphrodisiacs: The Science and the Myth'', Springer Science & Business Media, 2012, pp.100ff</ref> These preparations were known as ''cantharides'', from the Greek word for |
Preparations made from [[Spanish fly|blistering beetles]] have been used since ancient times as an [[aphrodisiac]], possibly because their physical effects were perceived to mimic those of sexual arousal,<ref>John L. Capinera, ''Encyclopedia of Entomology, Volume 4'', Springer Science & Business Media, 2008. p.2010</ref> and because they can cause prolonged erection or [[priapism]] in men.<ref name = aph>Peter V. Taberner, ''Aphrodisiacs: The Science and the Myth'', Springer Science & Business Media, 2012, pp.100ff</ref> These preparations were known as ''cantharides'', from the Greek word for “beetle.” |
||
Examples of such use found in historical sources include: |
Examples of such use found in historical sources include: |
||
* The ancient Roman historian [[Tacitus]] relates that a cantharid preparation was used by the empress [[Livia]], wife of [[Augustus Caesar]] to entice members of the imperial family or dinner guests to commit sexual indiscretions (thus providing her information to hold over them).<ref>{{cite book|title = Ancient Inventions|first = Peter|last = James|publisher = Ballantine Books|isbn = 978-0-345-40102-1|year = 1995|page = [https://archive.org/details/ancientinvention00jame/page/177 177]|url = https://archive.org/details/ancientinvention00jame/page/177}}</ref> |
* The ancient Roman historian [[Tacitus]] relates that a cantharid preparation was used by the empress [[Livia]], wife of [[Augustus Caesar]], to entice members of the imperial family or dinner guests to commit sexual indiscretions (thus, providing her information to hold over them).<ref>{{cite book|title = Ancient Inventions|first = Peter|last = James|publisher = Ballantine Books|isbn = 978-0-345-40102-1|year = 1995|page = [https://archive.org/details/ancientinvention00jame/page/177 177]|url = https://archive.org/details/ancientinvention00jame/page/177}}</ref> |
||
* The German emperor [[Henry IV, Holy Roman Emperor|Henry IV]] (1050–1106) is said to have consumed cantharides.<ref>{{cite web | url=http://blogs.scientificamerican.com/guest-blog/2012/03/13/when-sparks-fly-aphrodisiacs-and-the-fruit-fly/ | title=When Sparks Fly: Aphrodisiacs and the Fruit Fly | work=Scientific American | date=13 March 2012 | access-date=18 November 2014 | author=Eplett, Layla}}</ref> |
* The German emperor [[Henry IV, Holy Roman Emperor|Henry IV]] (1050–1106) is said to have consumed cantharides.<ref>{{cite web | url=http://blogs.scientificamerican.com/guest-blog/2012/03/13/when-sparks-fly-aphrodisiacs-and-the-fruit-fly/ | title=When Sparks Fly: Aphrodisiacs and the Fruit Fly | work=Scientific American | date=13 March 2012 | access-date=18 November 2014 | author=Eplett, Layla}}</ref> |
||
* The French surgeon [[Ambroise Paré]] (1510–1590) described a case in 1572 of a man suffering from |
* The French surgeon [[Ambroise Paré]] (1510–1590) described a case in 1572 of a man suffering from “the most frightful [[hypersexuality|satyriasis]]” after taking a potion composed of [[Urtica dioica|nettles]] and a cantharid extract.<ref name="Milsten_1">{{cite book |last=Milsten |first=Richard |title=The Sexual Male: Problems and Solutions|publisher=W. W. Norton & Company |year=2000 |isbn=978-0-393-32127-2 |page=170}}</ref> This is perhaps the same man of whom Paré relates that a [[courtesan]] sprinkled a cantharid powder on food she served to him, after which the man experienced "violent priapism" and anal bleeding, of which he later died. Paré also cites the case of a priest who died of [[hematuria]] after swallowing a dose of cantharides, which he intended to fortify his sex drive.<ref name = "Cabanès">[[Augustin Cabanès]], [https://archive.org/stream/b24878613#page/498/mode/2up ''Remèdes d'autrefois''], Paris: A. Maloine, 1910, p. 498ff.</ref> |
||
* Cantharides were in widespread use among the upper classes in France in the 1600s, despite being a banned substance. Police searches in connection with a rash of poisonings around 1680 turned up many stashes of |
* Cantharides were in widespread use among the upper classes in France in the 1600s, despite being a banned substance. Police searches in connection with a rash of poisonings around 1680 turned up many stashes of “bluish flies,” which were known to be used in the preparation of aphrodisiac potions.<ref name = "Cabanès"/> |
||
* The French sorceress [[La Voisin|Catherine Monvoisin]] (known as |
* The French sorceress [[La Voisin|Catherine Monvoisin]] (known as “La Voisin,” c. 1640–1680) was recorded in the 1670s as having prepared a love charm made from [[Spanish fly]] mixed with dried mole's blood and bat's blood.<ref>Richard Cavendish, ''The Black Arts'' (London: Pan Books, 1969), p. 333.<!--Can someone check the page number on this? It was inherited from a previous inaccurate form of the citation.--></ref> |
||
* Aphrodisiac sweets |
* Aphrodisiac sweets presumably laced with cantharides were circulated within [[libertine]] circles during the 1700s in France. They were multicolored tablets nicknamed “pastilles de Richelieu,” after the [[Armand de Vignerot du Plessis|Maréchal de Richelieu]], a notorious libertine (not to be confused with his great-uncle, the [[Cardinal Richelieu]]) who procured sexual encounters for King [[Louis XV]].<ref name = "Cabanès"/><ref>Jacques Levron, ''Le Maréchal de Richelieu, un libertin fastueux'' (Paris, Perrin, 1971).</ref> |
||
* The French writer Donatien Alphonse François — notoriously known as the [[Marquis de Sade]] (1740–1814) — is said to have given [[aniseed]]-flavored pastilles laced with Spanish fly to two prostitutes at a pair of orgies in 1772, poisoning and nearly killing them. He was sentenced to death for that (and for the crime of [[sodomy]]), but was later reprieved on appeal.<ref name="isbn0-394-54797-7">{{cite book |author1=Ford, Peter |author2=Howell, Michael |title=The beetle of Aphrodite and other medical mysteries |publisher=Random House |location=New York |year=1985 |isbn=978-0-394-54797-8 |url=https://archive.org/details/beetleofaphrodit00howe }}</ref><ref name = sch>Schaeffer, Neil (2000). ''The Marquis de Sade: A Life'', Cambridge, MA, USA: Harvard University Press, p. 58.</ref> |
* The French writer Donatien Alphonse François — notoriously known as the [[Marquis de Sade]] (1740–1814) — is said to have given [[aniseed]]-flavored pastilles laced with Spanish fly to two prostitutes at a pair of orgies in 1772, poisoning and nearly killing them. He was sentenced to death for that (and for the crime of [[sodomy]]), but was later reprieved on appeal.<ref name="isbn0-394-54797-7">{{cite book |author1=Ford, Peter |author2=Howell, Michael |title=The beetle of Aphrodite and other medical mysteries |publisher=Random House |location=New York |year=1985 |isbn=978-0-394-54797-8 |url=https://archive.org/details/beetleofaphrodit00howe }}</ref><ref name = sch>Schaeffer, Neil (2000). ''The Marquis de Sade: A Life'', Cambridge, MA, USA: Harvard University Press, p. 58.</ref> |
||
{{clear left}} |
{{clear left}} |
||
| Line 128: | Line 128: | ||
===Non-aphrodisiac uses=== |
===Non-aphrodisiac uses=== |
||
* The Spanish clergyman [[Juan de Horozco y Covarrubias]] ([[:es:Juan de Horozco y Covarrubias|es]]) (c. 1540–1610) reported the use of blister beetles as a [[poison]] as well as an aphrodisiac.<ref name="Covarrubias_1">{{cite book |title =Tesoros de la lengua castellana o española |last=Covarrubias-Horozco |first = S. |publisher = Universidad de Navarra - Iberoamericana - Vervuert |year = 2006 }}</ref> |
* The Spanish clergyman [[Juan de Horozco y Covarrubias]] ([[:es:Juan de Horozco y Covarrubias|es]]) (c. 1540–1610) reported the use of blister beetles as a [[poison]] as well as an aphrodisiac.<ref name="Covarrubias_1">{{cite book |title =Tesoros de la lengua castellana o española |last=Covarrubias-Horozco |first = S. |publisher = Universidad de Navarra - Iberoamericana - Vervuert |year = 2006 }}</ref> |
||
* Preparations of dried blister beetles were at one time used as a treatment for [[smallpox]].<ref>{{cite book|author=Johann Friedrich Closs|title=A New Method of Curing the Small-pox ... with a Specimen of Miscellaneous Observations on Medical Subjects; from the Latin ... by a Physician|url=https://books.google.com/books?id=HOtbAAAAcAAJ&pg=PA10|year=1767|publisher=Hawes}} Cantharides referred to throughout the book.</ref> As late as 1892 [[Andrew Taylor Still]], the founder of [[osteopathy]], recommended inhaling a tincture of cantharidin as an effective preventative and treatment for smallpox, decrying [[vaccination]].<ref>Andrew Taylor Still, ''The philosophy and mechanical principles of osteopathy'', 1892, chapter 12: "Smallpox". The 1902 edition is [http://www.anatomy4beginners.com/resources/The%20Philosophy%20and%20Mechanical%20Principles%20of%20Osteopathy%20AT%20Still%201902.pdf available here] {{Webarchive|url=https://web.archive.org/web/20190714030929/https://anatomy4beginners.com/resources/The%20Philosophy%20and%20Mechanical%20Principles%20of%20Osteopathy%20AT%20Still%201902.pdf |date=2019-07-14 }}.</ref> |
* Preparations of dried blister beetles were at one time used as a treatment for [[smallpox]].<ref>{{cite book|author=Johann Friedrich Closs|title=A New Method of Curing the Small-pox ... with a Specimen of Miscellaneous Observations on Medical Subjects; from the Latin ... by a Physician|url=https://books.google.com/books?id=HOtbAAAAcAAJ&pg=PA10|year=1767|publisher=Hawes}} Cantharides referred to throughout the book.</ref> As late as 1892, [[Andrew Taylor Still]], the founder of [[osteopathy]], recommended inhaling a [[tincture]] of cantharidin as an effective preventative and treatment for smallpox, decrying [[vaccination]].<ref>Andrew Taylor Still, ''The philosophy and mechanical principles of osteopathy'', 1892, chapter 12: "Smallpox". The 1902 edition is [http://www.anatomy4beginners.com/resources/The%20Philosophy%20and%20Mechanical%20Principles%20of%20Osteopathy%20AT%20Still%201902.pdf available here] {{Webarchive|url=https://web.archive.org/web/20190714030929/https://anatomy4beginners.com/resources/The%20Philosophy%20and%20Mechanical%20Principles%20of%20Osteopathy%20AT%20Still%201902.pdf |date=2019-07-14 }}.</ref> |
||
===Pharmaco-chemical isolation=== |
===Pharmaco-chemical isolation=== |
||
Cantharidin was first isolated as a chemically pure substance in 1810 by [[Pierre Robiquet]],<ref>{{Cite book | last = Wolter | first = H. | year = 1995 | title = Kompendium der Tierärztlichen Homöopathie | publisher = Enke | isbn = 978-3432978925 }}</ref> a French chemist then living in Paris. Robiquet isolated cantharidin as the active ingredient in pharmacological preparations of ''[[Lytta vesicatoria]]'', a.k.a. |
Cantharidin was first isolated as a chemically pure substance in 1810 by [[Pierre Robiquet]],<ref>{{Cite book | last = Wolter | first = H. | year = 1995 | title = Kompendium der Tierärztlichen Homöopathie | publisher = Enke | isbn = 978-3432978925 }}</ref> a French chemist then living in [[Paris]]. Robiquet isolated cantharidin as the active ingredient in pharmacological preparations of ''[[Lytta vesicatoria]]'', a.k.a. “[[Spanish fly]],” a species of [[blister beetle]]. This was one of the first historical instances of the identification and extraction of a simple active principle from a complex medicine. |
||
Robiquet found cantharidin to be an odorless and colorless solid at [[room temperature]]. He demonstrated that it was the active principle responsible for the aggressively [[blister agent|blistering properties]] of the coating of the eggs of the blister beetle, and established |
Robiquet found cantharidin to be an odorless and colorless solid at [[room temperature]]. He demonstrated that it was the active principle responsible for the aggressively [[blister agent|blistering properties]] of the coating of the eggs of the blister beetle, and additionally established that cantharidin had toxic properties comparable in degree to those of the most virulent poisons known in the 19th century, such as [[strychnine]].<ref>{{cite journal | last = Robiquet | first = P. J. | title = Expériences sur les cantharides | journal = Annales de Chimie | year = 1810 | volume = 76 | pages = 302–322 }}</ref> |
||
===Other uses of the pharmacological isolate=== |
===Other uses of the pharmacological isolate=== |
||
* Diluted solutions of cantharidin can be used as a [[topical]] medication to remove [[wart]]s<ref name="pmid13519856">{{cite journal |author1=Epstein, W. L. |author2=Kligman, A. M. | title = Treatment of warts with cantharidin | journal = AMA Archives of Dermatology | year = 1958 | volume = 77 | issue = 5 | pages = 508–511 | pmid = 13519856 | doi=10.1001/archderm.1958.01560050014003}}</ref><ref name="bacelieri">{{cite journal |author1=Bacelieri, R. |author2=Johnson, S. M. | title = Cutaneous warts: An evidence-based approach to therapy | journal = American Family Physician | year = 2005 | volume = 72 | issue = 4 | pages = 647–652 | pmid = 16127954 | url = http://www.aafp.org/afp/20050815/647.html }}</ref> and [[tattoo]]s and to treat the small [[papules]] of ''[[molluscum contagiosum]]''.<ref>{{cite web | title = Molluscum contagiosum | url = http://www.merck.com/mmpe/sec10/ch122/ch122b.html |date=November 2005 | publisher = Merck Manuals | access-date = 2007-10-21 }}</ref> |
* Diluted solutions of cantharidin can be used as a [[topical]] medication to remove [[wart]]s<ref name="pmid13519856">{{cite journal |author1=Epstein, W. L. |author2=Kligman, A. M. | title = Treatment of warts with cantharidin | journal = AMA Archives of Dermatology | year = 1958 | volume = 77 | issue = 5 | pages = 508–511 | pmid = 13519856 | doi=10.1001/archderm.1958.01560050014003}}</ref><ref name="bacelieri">{{cite journal |author1=Bacelieri, R. |author2=Johnson, S. M. | title = Cutaneous warts: An evidence-based approach to therapy | journal = American Family Physician | year = 2005 | volume = 72 | issue = 4 | pages = 647–652 | pmid = 16127954 | url = http://www.aafp.org/afp/20050815/647.html }}</ref> and [[tattoo]]s, and to treat the small [[papules]] of ''[[molluscum contagiosum]]''.<ref>{{cite web | title = Molluscum contagiosum | url = http://www.merck.com/mmpe/sec10/ch122/ch122b.html |date=November 2005 | publisher = Merck Manuals | access-date = 2007-10-21 }}</ref> |
||
* In [[Santería]] rituals, cantharides are used in [[incense]].<ref name="Gonzalez-Wippler_1">{{cite book |last=Gonzalez-Wippler |first=Migene |title=Santería: The Religion |publisher=Llewellyn Publications |year=2002 |isbn=978-1-56718-329-0 |page=[https://archive.org/details/santerareligio00gonz/page/221 221] |url=https://archive.org/details/santerareligio00gonz/page/221 }}</ref> |
* In [[Santería]] rituals, cantharides are used in [[incense]].<ref name="Gonzalez-Wippler_1">{{cite book |last=Gonzalez-Wippler |first=Migene |title=Santería: The Religion |publisher=Llewellyn Publications |year=2002 |isbn=978-1-56718-329-0 |page=[https://archive.org/details/santerareligio00gonz/page/221 221] |url=https://archive.org/details/santerareligio00gonz/page/221 }}</ref> |
||
==Veterinary issues== |
==Veterinary issues== |
||
Poisoning from cantharidin is a significant veterinary concern, especially in horses |
Poisoning by ''Epicauta'' species from cantharidin is a significant veterinary concern, especially in horses; species infesting feedstocks depend on region—e.g., ''Epicauta pennsylvanica'' (black blisterbeetle) in the U.S. midwest; and ''E. occidentalis, temexia, and vittata'' species (striped blister beetles) in the U.S. southwest—where the concentrations of the agent in each can vary substantially.<ref name = veterinary/> Beetles feed on [[weed]]s, and occasionally move into crop fields used to produce livestock feeds (e.g., [[alfalfa]]), where they are found to cluster and find their way into [[hay bale|baled hay]], e.g., a single flake (4–5 in. section<ref>{{cite book |author1=Rockett, Jody |author2=Bosted, Susanna | year = 2015 | title = Veterinary Clinical Procedures in Large Animal Practices | publisher = Cengage Learning | isbn = 978-1305537651 | location = Boston, MA, USA | url = https://books.google.com/books?isbn=1305537653 | access-date = 14 December 2015 | page = 65}}</ref>) may have several hundred insects, or none at all.<ref name = veterinary/> Horses are very sensitive to the cantharidin produced by beetle infestations: the {{LD50}} for horses is roughly 1 mg/kg of the horse’s body weight. Horses may be accidentally poisoned when fed bales of fodder with blister beetles in them.<ref>{{cite web | url = http://www.addl.purdue.edu/newsletters/2006/Fall/EquineCT.htm | title = Blister Beetle Poisoning / Cantharidin toxicosis | access-date = 2010-12-31 }}</ref> |
||
[[Great bustard]]s, a strongly polygynous bird species,<ref>{{cite journal|doi=10.1007/s00265-010-0972-6 |title=Correlates of male mating success in great bustard leks: the effects of age, weight, and display effort |year=2010 |last1=Alonso |first1=J.C. |last2=Magaña |first2=M. |last3=Palacín |first3=C. |last4=Martín |first4=C.A. |journal=Behavioral Ecology and Sociobiology |volume=64 |issue=10 |pages=1589–1600|hdl=10261/76985 |s2cid=8741416 }}</ref> are not immune to the toxicity of cantharidin; they become intoxicated after ingesting blister beetles |
[[Great bustard]]s, a strongly [[Polygyny|polygynous]] bird species,<ref>{{cite journal|doi=10.1007/s00265-010-0972-6 |title=Correlates of male mating success in great bustard leks: the effects of age, weight, and display effort |year=2010 |last1=Alonso |first1=J.C. |last2=Magaña |first2=M. |last3=Palacín |first3=C. |last4=Martín |first4=C.A. |journal=Behavioral Ecology and Sociobiology |volume=64 |issue=10 |pages=1589–1600|hdl=10261/76985 |s2cid=8741416 }}</ref> are not immune to the toxicity of cantharidin; they become intoxicated after ingesting blister beetles. However, cantharidin has activity also against parasites that infect them.<ref>{{cite journal|doi=10.1371/journal.pone.0111057 |pmid=25337911 |title=Males of a Strongly Polygynous Species Consume More Poisonous Food than Females |year=2014 |last1=Bravo |first1=C. |last2=Bautista |first2=L.M. |last3=García-París |first3=M. |last4=Blanco |first4=G. |last5=Alonso |first5=J.C. |journal=PLOS ONE |volume=9 |issue=10 |page=e111057 |pmc=4206510|bibcode=2014PLoSO...9k1057B |doi-access=free }}</ref><ref>{{cite journal|doi=10.1016/j.toxicon.2011.10.002 |pmid=22001622 |title=Possible cantharidin poisoning of a great bustard (Otis tarda) |year=2012 |last1=Sánchez-Barbudo |first1=I. S. |last2=Camarero |first2=P. |last3=García-Montijano |first3=M. |last4=Mateo |first4=R. |journal=Toxicon |volume=59 |issue=1 |pages=100–103|hdl=10261/143513 |hdl-access=free }}</ref> Great bustards may eat toxic [[blister beetle]]s of the genus ''[[Meloe]]'' to increase the sexual arousal of males.<ref name=Heneberg2016>{{cite journal |last1=Heneberg |first1=P. |title=On Otis tarda and Marquis de Sade: what motivates male Great Bustards to consume Blister Beetles (Meloidae)? |year=2016 |journal=Journal of Ornithology |volume=57 |issue=4 |pages=1123–1125 |doi=10.1007/s10336-016-1369-8|s2cid=17325635 }}</ref> |
||
==Human medical issues== |
==Human medical issues== |
||
===General risks=== |
===General risks=== |
||
As a blister agent, cantharidin has the potential to cause adverse effects when used medically; for this reason, it has been included in a list of |
As a blister agent, cantharidin has the potential to cause adverse effects when used medically; for this reason, it has been included in a list of “problem drugs” used by dermatologists<ref name="binder-1979">{{cite journal | author = Binder, R. | title = Malpractice--in dermatology | journal = Cutis; Cutaneous Medicine for the Practitioner | volume = 23 | issue = 5 | pages = 663–666 | year = 1979 | pmid = 456036 }}</ref> and emergency personnel.<ref name="karras-1996">{{cite journal | last1 = Karras | first1 = D. J. | last2 = Farrell | first2 = S. E. | last3 = Harrigan | first3 = R. A. | last4 = Henretig | first4 = F. M. | last5 = Gealt | first5 = L. | title = Poisoning from "Spanish fly" (cantharidin)| journal = The American Journal of Emergency Medicine | year = 1996 | volume = 14 | issue = 5 | pages = 478–483 | pmid = 8765116 | doi = 10.1016/S0735-6757(96)90158-8 | quote = While most commonly available preparations of Spanish fly contain cantharidin in negligible amounts, if at all, the chemical is available illicitly in concentrations capable of causing severe toxicity. Symptoms of cantharidin poisoning include burning of the mouth, dysphagia, nausea, hematemesis, gross hematuria, and dysuria. Mucosal erosion and hemorrhage is seen in the upper gastrointestinal (GI) tract. Renal dysfunction is common and related to acute tubular necrosis and glomerular destruction. }}</ref> |
||
Despite being widely used, cantharidin has never been and is not currently FDA approved.<ref>https://www.fda.gov/downloads/Drugs/.../PharmacyCompounding/UCM467373.pdf {{Bare URL PDF|date=March 2022}}</ref> |
Despite being widely used, cantharidin has never been and is not currently [[Food and Drug Administration|FDA]] approved.<ref>https://www.fda.gov/downloads/Drugs/.../PharmacyCompounding/UCM467373.pdf {{Bare URL PDF|date=March 2022}}</ref> It is currently in Phase 3 clinical trials for the treatment of [[Molluscum contagiosum|molluscum]].<ref>{{cite web|url=https://www.clinicaltrials.gov/ct2/show/NCT03377803?cond=molluscum&rank=1|title=Cantharidin Application in Molluscum Patients - Full Text View - ClinicalTrials.gov|date=28 May 2020}}</ref> However, when compounded properly and applied in the clinic topically by a medical provider familiar with its effects and uses, cantharidin can be safely and effectively used to treat some benign skin lesions, like warts and molluscum.<ref name=Moed2001/> |
||
When [[ingestion|ingested]] by humans, the {{LD50}} is around 0.5 mg/kg, with a dose of as little as 10 mg being potentially fatal. Ingesting cantharidin can initially cause severe damage to the lining of the gastrointestinal and urinary tracts, and may also cause permanent [[renal]] damage. Symptoms of cantharidin poisoning include [[hematuria|blood in the urine]], abdominal pain, and rarely [[priapism|prolonged erections]].<ref name=binder-1979/> |
When [[ingestion|ingested]] by humans, the {{LD50}} is around 0.5 mg/kg, with a dose of as little as 10 mg being potentially fatal. Ingesting cantharidin can initially cause severe damage to the lining of the [[Gastrointestinal tract|gastrointestinal]] and [[Urinary system|urinary tracts]], and may also cause permanent [[renal]] damage. Symptoms of cantharidin poisoning include [[hematuria|blood in the urine]], abdominal pain, and rarely [[priapism|prolonged erections]].<ref name=binder-1979/> |
||
===Risks of aphrodisiac use=== |
===Risks of aphrodisiac use=== |
||
| Line 160: | Line 160: | ||
===Mechanism of action=== |
===Mechanism of action=== |
||
{{missing information|section|toxicological mechanism when ingested|date=September 2022}} |
{{missing information|section|toxicological mechanism when ingested|date=September 2022}} |
||
Topical cantharidin is absorbed by the lipid membranes of epidermal cells, causing the release of [[serine proteases]], [[enzymes]] that break the [[peptide bonds]] in proteins. This causes the disintegration of [[Desmosome|desmosomal plaques]], cellular structures involved in cell-to-cell adhesion, leading to detachment of the [[Tonofibril|tonofilaments]] that hold cells together. The process leads to the loss of cellular connections ([[acantholysis]]) and ultimately blistering of the skin. Lesions heal without scarring.<ref name=Moed2001>{{Cite journal |author1=Moed, L. |author2=Shwayder, T. A. |author3=Chang, M. W. | title = Cantharidin revisited: A blistering defense of an ancient medicine | journal = Archives of Dermatology | year = 2001 | volume = 137 | issue = 10 | pages = 1357–1360 | pmid = 11594862 | doi=10.1001/archderm.137.10.1357| doi-access = free }}</ref><ref name=Bertaux1988>{{Cite journal |author1=Bertaux, B. |author2=Prost, C. |author3=Heslan, M. |author4=Dubertret, L. | title = Cantharide acantholysis: endogenous protease activation leading to desmosomal plaque dissolution | journal = British Journal of Dermatology | year = 1988 | volume = 118 | issue = 2 | pages = 157–165 | pmid = 3279999 | doi = 10.1111/j.1365-2133.1988.tb01769.x |s2cid=45714898 }}</ref> |
Topical cantharidin is absorbed by the lipid membranes of [[Epidermis|epidermal cells]], causing the release of [[serine proteases]], [[enzymes]] that break the [[peptide bonds]] in proteins. This causes the disintegration of [[Desmosome|desmosomal plaques]], cellular structures involved in cell-to-cell adhesion, leading to detachment of the [[Tonofibril|tonofilaments]] that hold cells together. The process leads to the loss of cellular connections ([[acantholysis]]), and ultimately results in blistering of the skin. Lesions heal without scarring.<ref name=Moed2001>{{Cite journal |author1=Moed, L. |author2=Shwayder, T. A. |author3=Chang, M. W. | title = Cantharidin revisited: A blistering defense of an ancient medicine | journal = Archives of Dermatology | year = 2001 | volume = 137 | issue = 10 | pages = 1357–1360 | pmid = 11594862 | doi=10.1001/archderm.137.10.1357| doi-access = free }}</ref><ref name=Bertaux1988>{{Cite journal |author1=Bertaux, B. |author2=Prost, C. |author3=Heslan, M. |author4=Dubertret, L. | title = Cantharide acantholysis: endogenous protease activation leading to desmosomal plaque dissolution | journal = British Journal of Dermatology | year = 1988 | volume = 118 | issue = 2 | pages = 157–165 | pmid = 3279999 | doi = 10.1111/j.1365-2133.1988.tb01769.x |s2cid=45714898 }}</ref> |
||
===Pharmaceutical use=== |
===Pharmaceutical use=== |
||
VP-102, an experimental drug-device combination |
VP-102, an experimental drug-device combination that includes cantharidin delivered via a single-use applicator, is being studied for the treatment of [[molluscum contagiosum]], [[wart|common warts]], and [[genital wart]]s.<ref>{{cite news | url = https://www.drugs.com/nda/vp_102_210528.html | title = Verrica Pharmaceuticals Announces Extension of FDA Review Period of its NDA for VP-102 for the Treatment of Molluscum Contagiosum | date = May 28, 2021 | publisher = [[drugs.com]] }}</ref> |
||
===Bioactivities=== |
===Bioactivities=== |
||
Revision as of 01:08, 2 January 2023

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,4S,7R,7aS)-3a,7a-Dimethylhexahydro-4,7-epoxy[2]benzofuran-1,3-dione | |
| Other names
Cantharidin, Spanish fly
| |
| Identifiers | |
3D model (JSmol)
|
|
| 85302 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.240 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O4 | |
| Molar mass | 196.202 g·mol−1 |
| Density | 1.41 g/cm3 |
| Melting point | 212 °C (414 °F; 485 K) |
| Pharmacology | |
| Legal status |
|
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H300, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
0.03–0.5 mg/kg (human) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cantharidin is an odorless, colorless fatty substance of the terpenoid class, which is secreted by many species of blister beetles.[a] It is a burn agent or a poison in large doses, but preparations containing it were historically used as aphrodisiacs (Spanish fly). In its natural form, cantharidin is secreted by the male blister beetle, and given to the female as a copulatory gift during mating. Afterwards, the female beetle covers her eggs with it as a defense against predators.
Poisoning from cantharidin is a significant veterinary concern, especially in horses, but it can also be poisonous to humans if taken internally (where the source is usually experimental self-exposure). Externally, cantharidin is a potent vesicant (blistering agent), exposure to which can cause severe chemical burns. Properly dosed and applied, the same properties have also been used therapeutically, for instance, for treatment of skin conditions, such as molluscum contagiosum infection of the skin.
Cantharidin is classified as an extremely hazardous substance in the United States, and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities.[1]
Chemistry
Structure and nomenclature
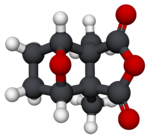
Cantharidin, from the Greek kantharis, for beetle,[2] is an odorless, colorless natural product with solubility in various organic solvents,[specify] but only slight solubility in water.[3] Its skeleton is tricyclic, formally, a tricyclo-[5.2.1.02,6]decane skeleton. Its functionalities include a carboxylic acid anhydride (−CO−O−CO−) substructure in one of its rings, as well as a bridging ether in its bicyclic ring system.

The complete mechanism of the biosynthesis of cantharidin is unknown. Its framework formally consists of two isoprene units.[4] However, feeding studies indicate that the biosynthetic process is more complicated, and not a simple product of geranyl pyrophosphate or related ten-carbon parent structure, as the seeming monoterpene nature would suggest. Instead, there is a farnesol (15-carbon) precursor from which certain carbon segments are later excised.[5]
Distribution and availability
The level of cantharidin in blister beetles can be quite variable. Among blister beetles of the genus Epicauta in Colorado, E. pennsylvanica contains about 0.2 mg, E. maculata contains 0.7 mg, and E. immaculata contains 4.8 mg per beetle; males also contain higher levels than females.[6]
Males of Berberomeloe majalis have higher level of cantharidin per beetle: 64.22 ± 51.28 mg/g (dry weight) and 9.10 ± 12.64 mg/g (d. w.). Cantharidin content in haemolymph is also higher in males (80.9 ± 106.5 µg/g) than in females (20.0 ± 41.5 µg/g).[7]
History

Aphrodisiac preparations
Preparations made from blistering beetles have been used since ancient times as an aphrodisiac, possibly because their physical effects were perceived to mimic those of sexual arousal,[8] and because they can cause prolonged erection or priapism in men.[9] These preparations were known as cantharides, from the Greek word for “beetle.”
Examples of such use found in historical sources include:
- The ancient Roman historian Tacitus relates that a cantharid preparation was used by the empress Livia, wife of Augustus Caesar, to entice members of the imperial family or dinner guests to commit sexual indiscretions (thus, providing her information to hold over them).[10]
- The German emperor Henry IV (1050–1106) is said to have consumed cantharides.[11]
- The French surgeon Ambroise Paré (1510–1590) described a case in 1572 of a man suffering from “the most frightful satyriasis” after taking a potion composed of nettles and a cantharid extract.[12] This is perhaps the same man of whom Paré relates that a courtesan sprinkled a cantharid powder on food she served to him, after which the man experienced "violent priapism" and anal bleeding, of which he later died. Paré also cites the case of a priest who died of hematuria after swallowing a dose of cantharides, which he intended to fortify his sex drive.[13]
- Cantharides were in widespread use among the upper classes in France in the 1600s, despite being a banned substance. Police searches in connection with a rash of poisonings around 1680 turned up many stashes of “bluish flies,” which were known to be used in the preparation of aphrodisiac potions.[13]
- The French sorceress Catherine Monvoisin (known as “La Voisin,” c. 1640–1680) was recorded in the 1670s as having prepared a love charm made from Spanish fly mixed with dried mole's blood and bat's blood.[14]
- Aphrodisiac sweets presumably laced with cantharides were circulated within libertine circles during the 1700s in France. They were multicolored tablets nicknamed “pastilles de Richelieu,” after the Maréchal de Richelieu, a notorious libertine (not to be confused with his great-uncle, the Cardinal Richelieu) who procured sexual encounters for King Louis XV.[13][15]
- The French writer Donatien Alphonse François — notoriously known as the Marquis de Sade (1740–1814) — is said to have given aniseed-flavored pastilles laced with Spanish fly to two prostitutes at a pair of orgies in 1772, poisoning and nearly killing them. He was sentenced to death for that (and for the crime of sodomy), but was later reprieved on appeal.[16][17]
Non-aphrodisiac uses
- The Spanish clergyman Juan de Horozco y Covarrubias (es) (c. 1540–1610) reported the use of blister beetles as a poison as well as an aphrodisiac.[18]
- Preparations of dried blister beetles were at one time used as a treatment for smallpox.[19] As late as 1892, Andrew Taylor Still, the founder of osteopathy, recommended inhaling a tincture of cantharidin as an effective preventative and treatment for smallpox, decrying vaccination.[20]
Pharmaco-chemical isolation
Cantharidin was first isolated as a chemically pure substance in 1810 by Pierre Robiquet,[21] a French chemist then living in Paris. Robiquet isolated cantharidin as the active ingredient in pharmacological preparations of Lytta vesicatoria, a.k.a. “Spanish fly,” a species of blister beetle. This was one of the first historical instances of the identification and extraction of a simple active principle from a complex medicine.
Robiquet found cantharidin to be an odorless and colorless solid at room temperature. He demonstrated that it was the active principle responsible for the aggressively blistering properties of the coating of the eggs of the blister beetle, and additionally established that cantharidin had toxic properties comparable in degree to those of the most virulent poisons known in the 19th century, such as strychnine.[22]
Other uses of the pharmacological isolate
- Diluted solutions of cantharidin can be used as a topical medication to remove warts[23][24] and tattoos, and to treat the small papules of molluscum contagiosum.[25]
- In Santería rituals, cantharides are used in incense.[26]
Veterinary issues
Poisoning by Epicauta species from cantharidin is a significant veterinary concern, especially in horses; species infesting feedstocks depend on region—e.g., Epicauta pennsylvanica (black blisterbeetle) in the U.S. midwest; and E. occidentalis, temexia, and vittata species (striped blister beetles) in the U.S. southwest—where the concentrations of the agent in each can vary substantially.[3] Beetles feed on weeds, and occasionally move into crop fields used to produce livestock feeds (e.g., alfalfa), where they are found to cluster and find their way into baled hay, e.g., a single flake (4–5 in. section[27]) may have several hundred insects, or none at all.[3] Horses are very sensitive to the cantharidin produced by beetle infestations: the LD50 for horses is roughly 1 mg/kg of the horse’s body weight. Horses may be accidentally poisoned when fed bales of fodder with blister beetles in them.[28]
Great bustards, a strongly polygynous bird species,[29] are not immune to the toxicity of cantharidin; they become intoxicated after ingesting blister beetles. However, cantharidin has activity also against parasites that infect them.[30][31] Great bustards may eat toxic blister beetles of the genus Meloe to increase the sexual arousal of males.[32]
Human medical issues
General risks
As a blister agent, cantharidin has the potential to cause adverse effects when used medically; for this reason, it has been included in a list of “problem drugs” used by dermatologists[33] and emergency personnel.[34] Despite being widely used, cantharidin has never been and is not currently FDA approved.[35] It is currently in Phase 3 clinical trials for the treatment of molluscum.[36] However, when compounded properly and applied in the clinic topically by a medical provider familiar with its effects and uses, cantharidin can be safely and effectively used to treat some benign skin lesions, like warts and molluscum.[37]
When ingested by humans, the LD50 is around 0.5 mg/kg, with a dose of as little as 10 mg being potentially fatal. Ingesting cantharidin can initially cause severe damage to the lining of the gastrointestinal and urinary tracts, and may also cause permanent renal damage. Symptoms of cantharidin poisoning include blood in the urine, abdominal pain, and rarely prolonged erections.[33]
Risks of aphrodisiac use
The extreme toxicity of cantharidin makes any use as an aphrodisiac highly dangerous.[38][39] As a result, it is illegal to sell (or use) cantharidin or preparations containing it without a prescription in many countries.[34]
Research
Mechanism of action
This section is missing information about toxicological mechanism when ingested. (September 2022) |
Topical cantharidin is absorbed by the lipid membranes of epidermal cells, causing the release of serine proteases, enzymes that break the peptide bonds in proteins. This causes the disintegration of desmosomal plaques, cellular structures involved in cell-to-cell adhesion, leading to detachment of the tonofilaments that hold cells together. The process leads to the loss of cellular connections (acantholysis), and ultimately results in blistering of the skin. Lesions heal without scarring.[37][40]
Pharmaceutical use
VP-102, an experimental drug-device combination that includes cantharidin delivered via a single-use applicator, is being studied for the treatment of molluscum contagiosum, common warts, and genital warts.[41]
Bioactivities
Cantharidin appears to have some effect in the topical treatment of cutaneous leishmaniasis in animal models.[42] In addition to topical medical applications, cantharidin and its analogues may have activity against cancer cells.[43][44][45] Laboratory studies with cultured tumor cells suggest that this activity may be the result of PP2A inhibition.[46][47]
References
Explanatory notes
- ^ Including broadly in genus Epicauta, genus Berberomeloe, and in species Lytta vesicatoria (Spanish fly). False blister beetles, cardinal beetles, and soldier beetles also produce cantharidin.
Sources
- ^ As defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002). See "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" Archived 2012-02-25 at the Wayback Machine (PDF) (July 1, 2008 ed.). U.S. Government Printing Office. Retrieved October 29, 2011.
- ^ A Dictionary of Entomology. CABI. 2011. p. 253.
- ^ a b c Schmitz, David G. (2013). "Overview of Cantharidin Poisoning (Blister Beetle Poisoning)". In Aiello, Susan E.; Moses, Michael A. (eds.). The Merck Veterinary Manual. Kenilworth, NJ, USA: Merck Sharp & Dohme. ISBN 978-0911910612. Retrieved 14 December 2015.
- ^ Richard J. Petroski, Susan P. McCormick (2012). Secondary-Metabolite Biosynthesis and Metabolism. Springer Science & Business Media.
- ^ Jiang, Ming; Lü, Shumin; Zhang, Yalin (2017). "The Potential Organ Involved in Cantharidin Biosynthesis in Epicauta chinensis Laporte (Coleoptera: Meloidae)". J Insect Sci. 17 (2): 52. doi:10.1093/jisesa/iex021. PMC 5633858. PMID 28423415.
- ^ Capinera, J. L.; Gardner, D. R.; Stermitz, F. R. (1985). "Cantharidin Levels in Blister Beetles (Coleoptera: Meloidae) Associated with Alfalfa in Colorado". Journal of Economic Entomology. 78 (5): 1052–1055. doi:10.1093/jee/78.5.1052.
- ^ Bravo, C.; Mas-Peinado, P.; Bautista, L. M.; Blanco, G.; Alonso, J. C.; García-París, M. (2017). "Cantharidin is conserved across phylogeographic lineages and present in both morphs of Iberian Berberomeloe blister beetles (Coleoptera, Meloidae)". Zoological Journal of the Linnean Society. 180 (4): 790–804. doi:10.1093/zoolinnean/zlw016. hdl:10261/153832.
- ^ John L. Capinera, Encyclopedia of Entomology, Volume 4, Springer Science & Business Media, 2008. p.2010
- ^ Peter V. Taberner, Aphrodisiacs: The Science and the Myth, Springer Science & Business Media, 2012, pp.100ff
- ^ James, Peter (1995). Ancient Inventions. Ballantine Books. p. 177. ISBN 978-0-345-40102-1.
- ^ Eplett, Layla (13 March 2012). "When Sparks Fly: Aphrodisiacs and the Fruit Fly". Scientific American. Retrieved 18 November 2014.
- ^ Milsten, Richard (2000). The Sexual Male: Problems and Solutions. W. W. Norton & Company. p. 170. ISBN 978-0-393-32127-2.
- ^ a b c Augustin Cabanès, Remèdes d'autrefois, Paris: A. Maloine, 1910, p. 498ff.
- ^ Richard Cavendish, The Black Arts (London: Pan Books, 1969), p. 333.
- ^ Jacques Levron, Le Maréchal de Richelieu, un libertin fastueux (Paris, Perrin, 1971).
- ^ Ford, Peter; Howell, Michael (1985). The beetle of Aphrodite and other medical mysteries. New York: Random House. ISBN 978-0-394-54797-8.
- ^ Schaeffer, Neil (2000). The Marquis de Sade: A Life, Cambridge, MA, USA: Harvard University Press, p. 58.
- ^ Covarrubias-Horozco, S. (2006). Tesoros de la lengua castellana o española. Universidad de Navarra - Iberoamericana - Vervuert.
- ^ Johann Friedrich Closs (1767). A New Method of Curing the Small-pox ... with a Specimen of Miscellaneous Observations on Medical Subjects; from the Latin ... by a Physician. Hawes. Cantharides referred to throughout the book.
- ^ Andrew Taylor Still, The philosophy and mechanical principles of osteopathy, 1892, chapter 12: "Smallpox". The 1902 edition is available here Archived 2019-07-14 at the Wayback Machine.
- ^ Wolter, H. (1995). Kompendium der Tierärztlichen Homöopathie. Enke. ISBN 978-3432978925.
- ^ Robiquet, P. J. (1810). "Expériences sur les cantharides". Annales de Chimie. 76: 302–322.
- ^ Epstein, W. L.; Kligman, A. M. (1958). "Treatment of warts with cantharidin". AMA Archives of Dermatology. 77 (5): 508–511. doi:10.1001/archderm.1958.01560050014003. PMID 13519856.
- ^ Bacelieri, R.; Johnson, S. M. (2005). "Cutaneous warts: An evidence-based approach to therapy". American Family Physician. 72 (4): 647–652. PMID 16127954.
- ^ "Molluscum contagiosum". Merck Manuals. November 2005. Retrieved 2007-10-21.
- ^ Gonzalez-Wippler, Migene (2002). Santería: The Religion. Llewellyn Publications. p. 221. ISBN 978-1-56718-329-0.
- ^ Rockett, Jody; Bosted, Susanna (2015). Veterinary Clinical Procedures in Large Animal Practices. Boston, MA, USA: Cengage Learning. p. 65. ISBN 978-1305537651. Retrieved 14 December 2015.
- ^ "Blister Beetle Poisoning / Cantharidin toxicosis". Retrieved 2010-12-31.
- ^ Alonso, J.C.; Magaña, M.; Palacín, C.; Martín, C.A. (2010). "Correlates of male mating success in great bustard leks: the effects of age, weight, and display effort". Behavioral Ecology and Sociobiology. 64 (10): 1589–1600. doi:10.1007/s00265-010-0972-6. hdl:10261/76985. S2CID 8741416.
- ^ Bravo, C.; Bautista, L.M.; García-París, M.; Blanco, G.; Alonso, J.C. (2014). "Males of a Strongly Polygynous Species Consume More Poisonous Food than Females". PLOS ONE. 9 (10): e111057. Bibcode:2014PLoSO...9k1057B. doi:10.1371/journal.pone.0111057. PMC 4206510. PMID 25337911.
- ^ Sánchez-Barbudo, I. S.; Camarero, P.; García-Montijano, M.; Mateo, R. (2012). "Possible cantharidin poisoning of a great bustard (Otis tarda)". Toxicon. 59 (1): 100–103. doi:10.1016/j.toxicon.2011.10.002. hdl:10261/143513. PMID 22001622.
- ^ Heneberg, P. (2016). "On Otis tarda and Marquis de Sade: what motivates male Great Bustards to consume Blister Beetles (Meloidae)?". Journal of Ornithology. 57 (4): 1123–1125. doi:10.1007/s10336-016-1369-8. S2CID 17325635.
- ^ a b Binder, R. (1979). "Malpractice--in dermatology". Cutis; Cutaneous Medicine for the Practitioner. 23 (5): 663–666. PMID 456036.
- ^ a b Karras, D. J.; Farrell, S. E.; Harrigan, R. A.; Henretig, F. M.; Gealt, L. (1996). "Poisoning from "Spanish fly" (cantharidin)". The American Journal of Emergency Medicine. 14 (5): 478–483. doi:10.1016/S0735-6757(96)90158-8. PMID 8765116.
While most commonly available preparations of Spanish fly contain cantharidin in negligible amounts, if at all, the chemical is available illicitly in concentrations capable of causing severe toxicity. Symptoms of cantharidin poisoning include burning of the mouth, dysphagia, nausea, hematemesis, gross hematuria, and dysuria. Mucosal erosion and hemorrhage is seen in the upper gastrointestinal (GI) tract. Renal dysfunction is common and related to acute tubular necrosis and glomerular destruction.
- ^ https://www.fda.gov/downloads/Drugs/.../PharmacyCompounding/UCM467373.pdf [bare URL PDF]
- ^ "Cantharidin Application in Molluscum Patients - Full Text View - ClinicalTrials.gov". 28 May 2020.
- ^ a b Moed, L.; Shwayder, T. A.; Chang, M. W. (2001). "Cantharidin revisited: A blistering defense of an ancient medicine". Archives of Dermatology. 137 (10): 1357–1360. doi:10.1001/archderm.137.10.1357. PMID 11594862.
- ^ Shamloul, R. (2010). "Natural aphrodisiacs". The Journal of Sexual Medicine. 7 (1 Pt 1): 39–49. doi:10.1111/j.1743-6109.2009.01521.x. PMID 19796015.
- ^ Sandroni, P. (2001). "Aphrodisiacs past and present: A historical review". Clinical Autonomic Research. 11 (5): 303–307. doi:10.1007/BF02332975. PMID 11758796. S2CID 32348540.
Cantharidin ("Spanish fly") is a chemical with vesicant properties derived from blister beetles, which has been used for millennia as a sexual stimulant by both sexes. Its mode of action is by inhibition of phosphodiesterase and protein phosphatase activity and stimulation of β-receptors, inducing vascular congestion and inflammation. Morbidity from its abuse is significant. The gastrointestinal tract sustains the brunt of toxicity, resulting in fatal hemorrhages. Renal toxicity is a result of its renal excretion, which may lead to acute tubular necrosis. Cardiac effects are most likely due to hemorrhagic shock, but they also can be due to myofibril degeneration, mitochondrial swelling, and pericardial and subendocardial hemorrhages.
- ^ Bertaux, B.; Prost, C.; Heslan, M.; Dubertret, L. (1988). "Cantharide acantholysis: endogenous protease activation leading to desmosomal plaque dissolution". British Journal of Dermatology. 118 (2): 157–165. doi:10.1111/j.1365-2133.1988.tb01769.x. PMID 3279999. S2CID 45714898.
- ^ "Verrica Pharmaceuticals Announces Extension of FDA Review Period of its NDA for VP-102 for the Treatment of Molluscum Contagiosum". drugs.com. May 28, 2021.
- ^ Ghaffarifar, F. (2010). "Leishmania major: In vitro and in vivo anti-leishmanial effect of cantharidin". Experimental Parasitology. 126 (2): 126–129. doi:10.1016/j.exppara.2010.04.004. PMID 20435039.
- ^ Ratcliffe, N. A.; Mello, C. B.; Garcia, E. S.; Butt, T. M.; Azambuja, P. (2011). "Insect natural products and processes: New treatments for human disease". Insect Biochemistry and Molecular Biology. 41 (10): 747–769. doi:10.1016/j.ibmb.2011.05.007. PMID 21658450.
- ^ Chen, Y. N.; Cheng, C. C.; Chen, J. C.; Tsauer, W.; Hsu, S. L. (2003). "Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells". British Journal of Pharmacology. 140 (3): 461–470. doi:10.1038/sj.bjp.0705461. PMC 1574052. PMID 12970086.
- ^ Zhang, C.; Peng, Y.; Wang, F.; Tan, X.; Liu, N.; Fan, S.; Wang, D.; Zhang, L.; Liu, D.; Wang, T.; Wang, S.; Zhou, Y.; Su, Y.; Cheng, T.; Zhuang, Z.; Shi, C. (2010). "A synthetic cantharidin analog for the enhancement of doxorubicin suppression of stem cell-derived aggressive sarcoma". Biomaterials. 31 (36): 9535–9543. doi:10.1016/j.biomaterials.2010.08.059. PMID 20875681.
- ^ Dorn, D. C.; Kou, C. A.; Png, K. J.; Moore, M. A. S. (2009). "The effect of cantharidins on leukemic stem cells". International Journal of Cancer. 124 (9): 2186–2199. doi:10.1002/ijc.24157. PMID 19123473. S2CID 38088568.
- ^ Li, W.; Xie, L.; Chen, Z.; Zhu, Y.; Sun, Y.; Miao, Y.; Xu, Z.; Han, X. (2010). "Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis". Cancer Science. 101 (5): 1226–1233. doi:10.1111/j.1349-7006.2010.01523.x. PMID 20331621. S2CID 24345174.
Further reading
- Dupuis, Gérard & Berland, Nicole (2004). "Cantharidin: Origin and synthesis," Lille, FR: Lycée Faidherbe, see [1], accessed 13 December 2015.

