T cell: Difference between revisions
more ref reformatting |
fix error - "thymocyte" is not another name for a T cell; & misc. minor |
||
| Line 18: | Line 18: | ||
| Code = {{TerminologiaHistologica|2|00|04.1.02007}} |
| Code = {{TerminologiaHistologica|2|00|04.1.02007}} |
||
}} |
}} |
||
'''T cells''' or '''T lymphocytes''' belong to a group of [[white blood cell]]s known as [[lymphocyte]]s, and play a central role in [[cell-mediated immunity]]. They can be distinguished from other lymphocytes, such as [[B cell]]s and [[natural killer cell]]s (NK cells), by the presence of a ''[[T cell receptor]]'' (TCR) on the cell surface. They are called ''T'' cells because they mature in the [[thymus]] |
'''T cells''' or '''T lymphocytes''' belong to a group of [[white blood cell]]s known as [[lymphocyte]]s, and play a central role in [[cell-mediated immunity]]. They can be distinguished from other lymphocytes, such as [[B cell]]s and [[natural killer cell]]s (NK cells), by the presence of a ''[[T cell receptor]]'' (TCR) on the cell surface. They are called ''T'' cells because they mature in the [[thymus]]. There are several subsets of T cells, each with a distinct function. |
||
== Types == |
== Types == |
||
| Line 45: | Line 45: | ||
=== Natural killer === |
=== Natural killer === |
||
'''[[Natural killer T cell]]s''' (NKT cells |
'''[[Natural killer T cell]]s''' (NKT cells – not to be confused with [[natural killer cell]]s) bridge the [[adaptive immune system]] with the [[innate immune system]]. Unlike conventional T cells that recognize peptide antigens presented by [[major histocompatibility complex]] (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called [[CD1d]]. Once activated, these cells can perform functions ascribed to both T<sub>h</sub> and T<sub>c</sub> cells (i.e., cytokine production and release of cytolytic/cell killing molecules). They are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses.{{citation needed|date=November 2011}} |
||
== γδ == |
|||
'''[[γδ T cells]]''' ([[gamma]] [[Delta (letter)|delta]] T cells) represent a small subset of T cells that possess a distinct [[T cell receptor]] (TCR) on their surface. A majority of T cells have a [[T cell receptor|TCR]] composed of two [[glycoprotein]] chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common (2% of total T cells) than the αβ T cells, but are found at their highest abundance in the gut [[mucosa]], within a population of lymphocytes known as [[intraepithelial lymphocyte]]s (IELs). The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on antigen presenting cells. Some murine γδ T cells recognize MHC class IB molecules though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of non-peptidic phosphorylated [[isoprenoid]] precursors, collectively named [[phosphoantigen]]s. Phosphoantigens are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are [[isopentenyl pyrophosphate]] (IPP) and its isomer [[dimethylallyl pyrophosphate]] (DMAPP). Many microbes produce the highly active compound hydroxy-DMAPP ([[HMB-PP]]) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and [[bisphosphonates|aminobisphosphonates]], which up-regulate endogenous IPP/DMAPP. |
'''[[γδ T cells]]''' ([[gamma]] [[Delta (letter)|delta]] T cells) represent a small subset of T cells that possess a distinct [[T cell receptor]] (TCR) on their surface. A majority of T cells have a [[T cell receptor|TCR]] composed of two [[glycoprotein]] chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common (2% of total T cells) than the αβ T cells, but are found at their highest abundance in the gut [[mucosa]], within a population of lymphocytes known as [[intraepithelial lymphocyte]]s (IELs). The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on antigen presenting cells. Some murine γδ T cells recognize MHC class IB molecules though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of non-peptidic phosphorylated [[isoprenoid]] precursors, collectively named [[phosphoantigen]]s. Phosphoantigens are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are [[isopentenyl pyrophosphate]] (IPP) and its isomer [[dimethylallyl pyrophosphate]] (DMAPP). Many microbes produce the highly active compound hydroxy-DMAPP ([[HMB-PP]]) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and [[bisphosphonates|aminobisphosphonates]], which up-regulate endogenous IPP/DMAPP. |
||
| Line 61: | Line 61: | ||
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3%<ref name="pmid10837068">{{cite journal | author = Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP | title = The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection | journal = Annu. Rev. Immunol. | volume = 18 | issue = | pages = 529–60 | year = 2000 | pmid = 10837068 | doi = 10.1146/annurev.immunol.18.1.529 }}</ref> a year throughout middle age, there is a corresponding fall in the thymic production of [[naive T cells]], leaving peripheral T cell expansion to play a greater role in protecting older subjects. |
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3%<ref name="pmid10837068">{{cite journal | author = Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP | title = The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection | journal = Annu. Rev. Immunol. | volume = 18 | issue = | pages = 529–60 | year = 2000 | pmid = 10837068 | doi = 10.1146/annurev.immunol.18.1.529 }}</ref> a year throughout middle age, there is a corresponding fall in the thymic production of [[naive T cells]], leaving peripheral T cell expansion to play a greater role in protecting older subjects. |
||
=== Beta |
=== Beta selection === |
||
{{Unreferenced section|date=June 2010}} |
{{Unreferenced section|date=June 2010}} |
||
Common lymphoid precursor cells that arrive at the thymus and become known as T-cell precursors. When they begin to express [[c-kit]] and [[CD44]], they become known as DN1 thymocytes. At this stage they must create a unique T-cell receptor through [[V(D)J recombination]]. As the TCRβ locus begins to be rearranged the DN1 cell begins to express CD25 and becomes a DN2 thymocyte. At this point a T-cell will either progress towards the Helper/Killer linage or the [[γδ T cell]] lineage. The DN2 cell begins to express [[CD3 (immunology)|CD3]] and stops expressing c-kit and CD44 becoming a DN3 thymocyte. At this stage the thymocyte must produce a TCRβ beta chain that can be translated into protein and travel to the cell surface with a Pre-T alpha chain. If this occurs, the cell has passed beta selection and will stop expressing CD25. It will also rapidly proliferate. The cell will start to express both CD4 and CD8 and becomes known as a DP thymocyte. |
Common lymphoid precursor cells that arrive at the thymus and become known as T-cell precursors. When they begin to express [[c-kit]] and [[CD44]], they become known as DN1 thymocytes. At this stage they must create a unique T-cell receptor through [[V(D)J recombination]]. As the TCRβ locus begins to be rearranged the DN1 cell begins to express CD25 and becomes a DN2 thymocyte. At this point a T-cell will either progress towards the Helper/Killer linage or the [[γδ T cell]] lineage. The DN2 cell begins to express [[CD3 (immunology)|CD3]] and stops expressing c-kit and CD44 becoming a DN3 thymocyte. At this stage the thymocyte must produce a TCRβ beta chain that can be translated into protein and travel to the cell surface with a Pre-T alpha chain. If this occurs, the cell has passed beta selection and will stop expressing CD25. It will also rapidly proliferate. The cell will start to express both CD4 and CD8 and becomes known as a DP thymocyte. |
||
| Line 83: | Line 83: | ||
Activation of CD4+ T cells occurs through the engagement of both the [[T cell receptor]] and [[CD28]] on the T cell by the [[major histocompatibility complex]] (MHC) [[peptide]] and [[B7 (protein)|B7]] family members on the [[antigen-presenting cell|APC]], respectively. Both are required for production of an effective immune response; in the absence of CD28 [[co-stimulation]], T-cell receptor signalling alone results in [[anergy]]. The signalling pathways downstream from both CD28 and the T cell receptor involve many proteins. |
Activation of CD4+ T cells occurs through the engagement of both the [[T cell receptor]] and [[CD28]] on the T cell by the [[major histocompatibility complex]] (MHC) [[peptide]] and [[B7 (protein)|B7]] family members on the [[antigen-presenting cell|APC]], respectively. Both are required for production of an effective immune response; in the absence of CD28 [[co-stimulation]], T-cell receptor signalling alone results in [[anergy]]. The signalling pathways downstream from both CD28 and the T cell receptor involve many proteins. |
||
The first signal is provided by binding of the T cell receptor to a short peptide presented by the major histocompatibility complex (MHC) on another cell. This ensures that only a T cell with a TCR specific to that peptide is activated. The partner cell is usually a professional antigen presenting cell (APC), usually a [[dendritic cell]] in the case of [[naive T cell|naïve]] responses, although B cells and macrophages can be important APCs. The peptides presented to [[CD8]]<sup>+</sup> T cells by MHC class I molecules are |
The first signal is provided by binding of the T cell receptor to a short peptide presented by the major histocompatibility complex (MHC) on another cell. This ensures that only a T cell with a TCR specific to that peptide is activated. The partner cell is usually a professional antigen presenting cell (APC), usually a [[dendritic cell]] in the case of [[naive T cell|naïve]] responses, although B cells and macrophages can be important APCs. The peptides presented to [[CD8]]<sup>+</sup> T cells by MHC class I molecules are 8–9 amino acids in length; the peptides presented to [[CD4]]<sup>+</sup> cells by [[Major histocompatibility complex|MHC]] class II molecules are longer, usually 12–25 amino acids in length,<ref>Jennifer Rolland and Robyn O'Hehir, "Turning off the T-cells: Peptides for treatment of allergic Diseases," Today's life science publishing, 1999, Page 32</ref> as the ends of the binding cleft of the MHC class II molecule are open. |
||
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as [[necrosis|necrotic]]-bodies or [[heat shock proteins]]. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the [[CD80]] and [[CD86]] proteins, which together constitute the [[B7 (protein)|B7]] protein, (B7.1 and B7.2 respectively) on the APC. Other receptors are expressed upon activation of the T cell, such as [[OX40]] and [[CD278|ICOS]], but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes [[anergy|anergic]], and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation. |
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as [[necrosis|necrotic]]-bodies or [[heat shock proteins]]. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the [[CD80]] and [[CD86]] proteins, which together constitute the [[B7 (protein)|B7]] protein, (B7.1 and B7.2 respectively) on the APC. Other receptors are expressed upon activation of the T cell, such as [[OX40]] and [[CD278|ICOS]], but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes [[anergy|anergic]], and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation. |
||
| Line 120: | Line 120: | ||
* [http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=2 Immunobiology, 5th Edition] |
* [http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=2 Immunobiology, 5th Edition] |
||
* [http://www.niaid.nih.gov/publications/immune/the_immune_system.pdf niaid.nih.gov] – The Immune System |
* [http://www.niaid.nih.gov/publications/immune/the_immune_system.pdf niaid.nih.gov] – The Immune System |
||
* [http://www.tcells.org T-cell Group |
* [http://www.tcells.org T-cell Group – Cardiff University] |
||
* [http://content.nejm.org/cgi/content/full/358/25/2698 (Successful!) Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1]. |
* [http://content.nejm.org/cgi/content/full/358/25/2698 (Successful!) Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1]. |
||
* [http://www.modelingimmunity.org The Center for Modeling Immunity to Enteric Pathogens (MIEP)] |
* [http://www.modelingimmunity.org The Center for Modeling Immunity to Enteric Pathogens (MIEP)] |
||
Revision as of 10:27, 21 May 2012
| T cell | |
|---|---|
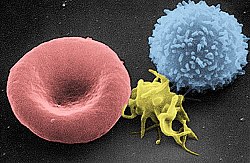 Scanning electron micrograph of T lymphocyte (right), a platelet (center) and a red blood cell (left) | |
| Details | |
| Identifiers | |
| Latin | lymphocytus T |
| MeSH | D013601 |
| TH | H2.00.04.1.02007 |
| FMA | 62870 |
| Anatomical terminology | |
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells (NK cells), by the presence of a T cell receptor (TCR) on the cell surface. They are called T cells because they mature in the thymus. There are several subsets of T cells, each with a distinct function.
Types
Helper
T helper cell (TH cells) assist other white blood cells in immunologic processes, including maturation of B cells into plasma cells and memory B cells, and activation of cytotoxic T cells and macrophages. These cells are also known as CD4+ T cells because they express the CD4 protein on their surface. Helper T cells become activated when they are presented with peptide antigens by MHC class II molecules, which are expressed on the surface of antigen presenting cells (APCs). Once activated, they divide rapidly and secrete small proteins called cytokines that regulate or assist in the active immune response. These cells can differentiate into one of several subtypes, including TH1, TH2, TH3, TH17, or TFH, which secrete different cytokines to facilitate a different type of immune response. Signalling from the APC directs T cells into particular subtypes.[1]
Cytotoxic
Cytotoxic T cells (TC cells, or CTLs) destroy virally infected cells and tumor cells, and are also implicated in transplant rejection. These cells are also known as CD8+ T cells since they express the CD8 glycoprotein at their surface. These cells recognize their targets by binding to antigen associated with MHC class I, which is present on the surface of nearly every cell of the body. Through IL-10, adenosine and other molecules secreted by regulatory T cells, the CD8+ cells can be inactivated to an anergic state, which prevent autoimmune diseases such as experimental autoimmune encephalomyelitis.[2]
Memory
Memory T cells are a subset of antigen-specific T cells that persist long-term after an infection has resolved. They quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen, thus providing the immune system with "memory" against past infections. Memory T cells comprise two subtypes: central memory T cells (TCM cells) and effector memory T cells (TEM cells). Memory cells may be either CD4+ or CD8+. Memory T cells typically express the cell surface protein CD45RO.[3]
Regulatory
Regulatory T cells (Treg cells), formerly known as suppressor T cells, are crucial for the maintenance of immunological tolerance. Their major role is to shut down T cell-mediated immunity toward the end of an immune reaction and to suppress auto-reactive T cells that escaped the process of negative selection in the thymus.
Two major classes of CD4+ Treg cells have been described — naturally occurring Treg cells and adaptive Treg cells.
Naturally occurring Treg cells (also known as CD4+CD25+FoxP3+ Treg cells) arise in the thymus and have been linked to interactions between developing T cells with both myeloid (CD11c+) and plasmacytoid (CD123+) dendritic cells that have been activated with TSLP.[4][5] Naturally occurring Treg cells can be distinguished from other T cells by the presence of an intracellular molecule called FoxP3. Mutations of the FOXP3 gene can prevent regulatory T cell development, causing the fatal autoimmune disease IPEX.
Adaptive Treg cells (also known as Tr1 cells or Th3 cells) may originate during a normal immune response.
Natural killer
Natural killer T cells (NKT cells – not to be confused with natural killer cells) bridge the adaptive immune system with the innate immune system. Unlike conventional T cells that recognize peptide antigens presented by major histocompatibility complex (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called CD1d. Once activated, these cells can perform functions ascribed to both Th and Tc cells (i.e., cytokine production and release of cytolytic/cell killing molecules). They are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses.[citation needed]
γδ
γδ T cells (gamma delta T cells) represent a small subset of T cells that possess a distinct T cell receptor (TCR) on their surface. A majority of T cells have a TCR composed of two glycoprotein chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common (2% of total T cells) than the αβ T cells, but are found at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs). The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on antigen presenting cells. Some murine γδ T cells recognize MHC class IB molecules though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of non-peptidic phosphorylated isoprenoid precursors, collectively named phosphoantigens. Phosphoantigens are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP). Many microbes produce the highly active compound hydroxy-DMAPP (HMB-PP) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and aminobisphosphonates, which up-regulate endogenous IPP/DMAPP.
Development in the thymus
See Thymocyte for review of thymic selection
All T cells originate from haematopoietic stem cells in the bone marrow. Haematopoietic progenitors derived from haematopoietic stem cells populate the thymus and expand by cell division to generate a large population of immature thymocytes.[6] The earliest thymocytes express neither CD4 nor CD8, and are therefore classed as double-negative (CD4-CD8-) cells. As they progress through their development they become double-positive thymocytes (CD4+CD8+), and finally mature to single-positive (CD4+CD8- or CD4-CD8+) thymocytes that are then released from the thymus to peripheral tissues.
About 98% of thymocytes die during the development processes in the thymus by failing either positive selection or negative selection, whereas the other 2% survive and leave the thymus to become mature immunocompetent T cells.
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3%[7] a year throughout middle age, there is a corresponding fall in the thymic production of naive T cells, leaving peripheral T cell expansion to play a greater role in protecting older subjects.
Beta selection
Common lymphoid precursor cells that arrive at the thymus and become known as T-cell precursors. When they begin to express c-kit and CD44, they become known as DN1 thymocytes. At this stage they must create a unique T-cell receptor through V(D)J recombination. As the TCRβ locus begins to be rearranged the DN1 cell begins to express CD25 and becomes a DN2 thymocyte. At this point a T-cell will either progress towards the Helper/Killer linage or the γδ T cell lineage. The DN2 cell begins to express CD3 and stops expressing c-kit and CD44 becoming a DN3 thymocyte. At this stage the thymocyte must produce a TCRβ beta chain that can be translated into protein and travel to the cell surface with a Pre-T alpha chain. If this occurs, the cell has passed beta selection and will stop expressing CD25. It will also rapidly proliferate. The cell will start to express both CD4 and CD8 and becomes known as a DP thymocyte.
Positive selection
Positive selection "selects for" T cells capable of interacting with MHC. Double-positive thymocytes (CD4+/CD8+) move deep into the thymic cortex where they are presented with self-antigens. These self-antigens are expressed by thymic cortical epithelial cells that express Activation-Induced (Cytidine) Deaminase on both MHC molecules on the surface of cortical cells. Only those thymocytes that interact with MHC-I or MHC-II will receive a vital "survival signal." All that can't will die by apoptosis. This process insures that the TCR on affinity cannot serve useful functions in the body (i.e. the cells must be able to interact with MHC and peptide complexes in order to effect immune responses). The vast majority of all thymocytes end up dying during this process.
A thymocyte's fate is also determined during positive selection. Double-positive cells (CD4+/CD8+) that are positively selected on MHC class II molecules will eventually become CD4+ cells, while cells positively selected on MHC class I molecules mature into CD8+ cells. A T cell becomes a CD4+ cell by downregulating expression of its CD8 cell surface receptors. If the cell does not lose its signal through the ITAM pathway, it will continue down-regulating CD8 and become a CD4+, single positive cell. But if there is a signal interruption, the cell stops downregulating CD8 and switches over to downregulating CD4 molecules instead, eventually becoming a CD8+, single positive cell.
This process does not remove thymocytes that may cause autoimmunity. The potentially autoimmune cells are removed by the process of negative selection (discussed below).
Negative selection
Negative selection removes thymocytes that are capable of strongly binding with "self" peptides presented by MHC. Thymocytes that survive positive selection migrate towards the boundary of the thymic cortex and thymic medulla. While in the medulla, they are again presented with self-antigen in complex with MHC molecules on thymic epithelial cells.[8] Thymocytes that interact too strongly with the antigen receive an apoptotic signal that leads to cell death. However, some of these cells are selected to become Treg cells. The remaining cells exit the thymus as mature naive T cells. This process is an important component of central tolerance and serves to prevent the formation of self-reactive T cells that are capable of inducing autoimmune diseases in the host.
In summary, β-selection is the first checkpoint, where the T cells which are able to form a functional TCRβ chain and bind to pre-Tα are allowed to proceed. Next, positive selection checks that T cells have successfully re-arranged their TCRα locus and are capable of recognizing peptide-MHC complexes. Negative selection then removes for T cells that bind too strongly to self antigens. These selection processes allow for tolerance of self by the immune system.
Activation

Activation of CD4+ T cells occurs through the engagement of both the T cell receptor and CD28 on the T cell by the major histocompatibility complex (MHC) peptide and B7 family members on the APC, respectively. Both are required for production of an effective immune response; in the absence of CD28 co-stimulation, T-cell receptor signalling alone results in anergy. The signalling pathways downstream from both CD28 and the T cell receptor involve many proteins.
The first signal is provided by binding of the T cell receptor to a short peptide presented by the major histocompatibility complex (MHC) on another cell. This ensures that only a T cell with a TCR specific to that peptide is activated. The partner cell is usually a professional antigen presenting cell (APC), usually a dendritic cell in the case of naïve responses, although B cells and macrophages can be important APCs. The peptides presented to CD8+ T cells by MHC class I molecules are 8–9 amino acids in length; the peptides presented to CD4+ cells by MHC class II molecules are longer, usually 12–25 amino acids in length,[9] as the ends of the binding cleft of the MHC class II molecule are open.
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as necrotic-bodies or heat shock proteins. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the CD80 and CD86 proteins, which together constitute the B7 protein, (B7.1 and B7.2 respectively) on the APC. Other receptors are expressed upon activation of the T cell, such as OX40 and ICOS, but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes anergic, and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation.
The T cell receptor exists as a complex of several proteins. The actual T cell receptor is composed of two separate peptide chains, which are produced from the independent T cell receptor alpha and beta (TCRα and TCRβ) genes. The other proteins in the complex are the CD3 proteins: CD3εγ and CD3εδ heterodimers and, most important, a CD3ζ homodimer, which has a total of six ITAM motifs. The ITAM motifs on the CD3ζ can be phosphorylated by Lck and in turn recruit ZAP-70. Lck and/or ZAP-70 can also phosphorylate the tyrosines on many other molecules, not least CD28, LAT and SLP-76, which allows the aggregation of signalling complexes around these proteins.
Phosphorylated LAT recruits SLP-76 to the membrane, where it can then bring in PLC-γ, VAV1, Itk and potentially PI3K. Both PLC-γ and PI3K act on PI(4,5)P2 on the inner leaflet of the membrane to create the active intermediaries diacylglycerol (DAG), inositol-1,4,5-trisphosphate (IP3), and phosphatidlyinositol-3,4,5-trisphosphate (PIP3). DAG binds and activates some PKCs, most important, in T cells PKCθ, a process important for activating the transcription factors NF-κB and AP-1. IP3 is released from the membrane by PLC-γ and diffuses rapidly to activate receptors on the ER, which induce the release of calcium. The released calcium then activates calcineurin, and calcineurin in turn activates NFAT, which then translocates to the nucleus. NFAT is a transcription factor, which activates the transcription of a pleiotropic set of genes, most notable, IL-2, a cytokine that promotes long term proliferation of activated T cells.
PLCγ can also initiate the NF-κB pathway. DAG activates PKCθ, which then phosphorylates CARMA1 causing it to unfold and function as a scaffold. The cytosolic domains bind an adapter Cbl 10 via CARD (Caspase activation and recruitment domains) domains; that then binds TRAF6, which is ubiquitinated at K63.Template:Rp:513–523[10] This form of ubiquitination does not lead to degradation of target proteins. Rather it serves to recruit NEMO, IKKα and β, and TAB1-2/ TAK1.[11] TAK 1 phosphorylates IKK-β, which then phosphorylates IκB allowing for K48 ubiquitination: leads to proteosomal degradation. Rel A and p50 can then enter the nucleus and bind the NF-κB response element. This coupled with NFAT signaling allows for complete activation of the IL-2 gene.[10]
While in most cases activation is dependent on TCR recognition of antigen, alternative pathways for activation have been described. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter.[12]
Disorders
Deficiency
Causes of T cell deficiency include lymphocytopenia of T cells and/or defects on function of individual T cells. Complete insufficiency of T cell function can result from hereditary conditions such as severe combined immunodeficiency (SCID), Omenn syndrome, and Cartilage-hair hypoplasia.[13] Causes of partial insufficiencies of T cell function include acquired immune deficiency syndrome (AIDS), and hereditary conditions such as DiGeorge syndrome (DGS), chromosomal breakage syndromes (CBSs), and B-cell and T-cell combined disorders such as ataxia telangiectasia (AT) and Wiskott-Aldrich syndrome (WAS).[13]
The main pathogens of concern in T cell deficiencies are intracellular pathogens, including Herpes simplex virus, Mycobacterium and Listeria.[14] Also, fungal infections are also more common and severe in T cell deficiencies.[14]
Cancer
Cancer of T cells is termed T-cell lymphoma, and accounts for perhaps one in ten cases of non-Hodgkin lymphoma.[15] The main forms of T cell lymphoma are:
- Extranodal T cell lymphoma
- Cutaneous T cell lymphomas: Sézary syndrome and Mycosis fungoides
- Anaplastic large cell lymphoma
- Angioimmunoblastic T cell lymphoma
See also
References
- ^ Journal of Clinical Investigation (2007-05-01). "APC-derived cytokines and T cell polarization in autoimmune inflammation". Jci.org. Retrieved 2012-04-09.
- ^ Jiang H, Chess L (2004). "An integrated view of suppressor T cell subsets in immunoregulation". J. Clin. Invest. 114 (9): 1198–208. doi:10.1172/JCI23411. PMC 524238. PMID 15520848.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Akbar AN, Terry L, Timms A, Beverley PC, Janossy G (1988). "Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells". J. Immunol. 140 (7): 2171–8. PMID 2965180.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ (2005). "Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus". Nature. 436 (7054): 1181–5. doi:10.1038/nature03886. PMID 16121185.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ (2010). "Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus". J. Immunol. 184 (6): 2999–3007. doi:10.4049/jimmunol.0804106. PMID 20173030.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schwarz BA, Bhandoola A (2006). "Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis". Immunol. Rev. 209: 47–57. doi:10.1111/j.0105-2896.2006.00350.x. PMID 16448533.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP (2000). "The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection". Annu. Rev. Immunol. 18: 529–60. doi:10.1146/annurev.immunol.18.1.529. PMID 10837068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L (2010). "Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance". Nat. Immunol. 11 (6): 512–9. doi:10.1038/ni.1874. PMID 20431619.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jennifer Rolland and Robyn O'Hehir, "Turning off the T-cells: Peptides for treatment of allergic Diseases," Today's life science publishing, 1999, Page 32
- ^ a b Tatham P, Gomperts BD, Kramer IM (2003). Signal transduction. Amsterdam: Elsevier Academic Press. ISBN 0-12-289632-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Wu H, Arron JR (2003). "TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology". Bioessays. 25 (11): 1096–105. doi:10.1002/bies.10352. PMID 14579250.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Milstein O, Hagin D, Lask A, Reich-Zeliger S, Shezen E, Ophir E, Eidelstein Y, Afik R, Antebi YE, Dustin ML, Reisner Y (2011). "CTLs respond with activation and granule secretion when serving as targets for T-cell recognition". Blood. 117 (3): 1042–52. doi:10.1182/blood-2010-05-283770. PMC 3035066. PMID 21045195.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Medscape > T-cell Disorders. Author: Robert A Schwartz, MD, MPH; Chief Editor: Harumi Jyonouchi, MD. Updated: May 16, 2011
- ^ a b Jones J, Bannister BA, Gillespie SH, ed. (2006). Infection: Microbiology and Management. Wiley-Blackwell. p. 435. ISBN 1-4051-2665-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ "The Lymphomas" (PDF). The Leukemia & Lymphoma Society. May 2006. p. 2. Retrieved 2008-04-07.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help)
