Xylitol: Difference between revisions
No edit summary |
Undid revision 296695779 by 75.87.137.127 (talk) |
||
| Line 24: | Line 24: | ||
[[Image:Xylitol crystals.jpg|thumb|right|Xylitol crystals]] |
[[Image:Xylitol crystals.jpg|thumb|right|Xylitol crystals]] |
||
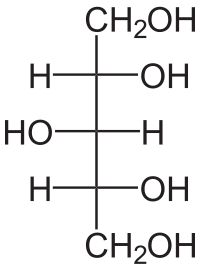
'''Xylitol''' (from Greek ξύλον - ''xyl''[''on''], "wood" + suffix -''itol'', used to denote sugar alcohols) is an [[organic compound]] with the formula (CHOH)<sub>3</sub>(CH<sub>2</sub>OH)<sub>2</sub>. This [[Chirality (chemistry)|achiral]] species is one of four [[isomer]]s of 1,2,3,4,5-pentapentanol. This [[sugar alcohol]] is used as a naturally occurring [[sugar substitute]] found in the [[fibre]]s of many [[fruit]]s and [[vegetable]]s, including various [[berry|berries]], [[corn husk]]s, [[oat]]s, and [[mushroom]]s.<ref>{{cite book |last=Gare |first=Fran |title=The Sweet Miracle of Xylitol |url=http://books.google.com/books?id=5tgZG6Sb2aAC |date=February 1, 2003 |publisher=Basic Health Publications, Inc. |isbn=1-59120-038-5 }}</ref> It can be extracted from corn fibre,<ref>R Sreenivas Rao, Ch. Pavanajyothi, RS Prakasham, PN Sharma, L Venkateswar Rao (2006) Xylitol production from corn fibre and sugarcane bagasse hydrolysates by Candida tropicalis ''Bioresource Technology'' 97:1974-1978.</ref> [[birch]], [[raspberry|raspberries]], [[plum]]s, and [[maize|corn]]. Xylitol is roughly as sweet as [[sucrose]] with only two-thirds the [[food energy]]. |
'''Xylitol''' (from Greek ξύλον - ''xyl''[''on''], "wood" + suffix -''itol'', used to denote sugar alcohols) is an [[organic compound]] with the formula (CHOH)<sub>3</sub>(CH<sub>2</sub>OH)<sub>2</sub>. This [[Chirality (chemistry)|achiral]] species is one of four [[isomer]]s of 1,2,3,4,5-pentapentanol. This [[sugar alcohol]] is used as a naturally occurring [[sugar substitute]] found in the [[fibre]]s of many [[fruit]]s and [[vegetable]]s, including various [[berry|berries]], [[corn husk]]s, [[oat]]s, and [[mushroom]]s.<ref>{{cite book |last=Gare |first=Fran |title=The Sweet Miracle of Xylitol |url=http://books.google.com/books?id=5tgZG6Sb2aAC |date=February 1, 2003 |publisher=Basic Health Publications, Inc. |isbn=1-59120-038-5 }}</ref> It can be extracted from corn fibre,<ref>R Sreenivas Rao, Ch. Pavanajyothi, RS Prakasham, PN Sharma, L Venkateswar Rao (2006) Xylitol production from corn fibre and sugarcane bagasse hydrolysates by Candida tropicalis ''Bioresource Technology'' 97:1974-1978.</ref> [[birch]], [[raspberry|raspberries]], [[plum]]s, and [[maize|corn]]. Xylitol is roughly as sweet as [[sucrose]] with only two-thirds the [[food energy]]. |
||
I don't think xylitol is a sugar alcohol. |
|||
==Production of xylitol== |
==Production of xylitol== |
||
Revision as of 05:23, 16 June 2009
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4S)-Pentane-1,2,3,4,5-pentol
| |
| Other names
1,2,3,4,5-Pentahydroxypentane
| |
| Identifiers | |
| ECHA InfoCard | 100.001.626 |
| E number | E967 (glazing agents, ...) |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C5H12O5 | |
| Molar mass | 152.15 g/mol |
| Density | 1.52 g/cm³ |
| Melting point | 92-96 °C |
| Boiling point | 216 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Xylitol (from Greek ξύλον - xyl[on], "wood" + suffix -itol, used to denote sugar alcohols) is an organic compound with the formula (CHOH)3(CH2OH)2. This achiral species is one of four isomers of 1,2,3,4,5-pentapentanol. This sugar alcohol is used as a naturally occurring sugar substitute found in the fibres of many fruits and vegetables, including various berries, corn husks, oats, and mushrooms.[2] It can be extracted from corn fibre,[3] birch, raspberries, plums, and corn. Xylitol is roughly as sweet as sucrose with only two-thirds the food energy.
Production of xylitol
Xylitol (Finnish ksylitoli) was first derived from birch trees in Finland in the 20th century and was first popularised in Europe as a safe sweetener for people with diabetes that would not impact insulin levels. Today, using hardwood or maize sources, the largest manufacturer globally is the Danish company Danisco, with several other suppliers from China.[4][5] Xylitol is produced by hydrogenation of xylose, which converts the sugar (an aldehyde) into a primary alcohol.
Properties
One teaspoon (5 mL) of xylitol contains 9.6 calories, as compared to one teaspoon of sugar, which has 15 calories. Xylitol has virtually no aftertaste, and is advertised as "safe for diabetics and individuals with hyperglycemia". This is because sugar-alcohols have less impact on a person's blood sugar than regular sugars.[6]
Dietary use worldwide
Xylitol is widely used in Finland, its "home country." Many Finnish confectioneries employ xylitol, or have a xylitol version available. Virtually all chewing gum sold in Finland is sweetened with xylitol.[7]
Medical applications
Dental care
Xylitol is a "tooth friendly" non-fermentable sugar alcohol[8][9]. A systematic review study[10] on the efficacy of Xylitol has indicated dental heath benefits in caries prevention, showing superior performance to other polyols. Early studies from Finland in the 1970s found that a group chewing sucrose gum had 2.92 decayed, missing, or filled (dmf) teeth compared to 1.04 in the group chewing xylitol gums.[11] In another study, researchers had mothers chew xylitol gum 3 months after delivery until their children were 2 years old. The researchers found that the xylitol group had "a 70% reduction in cavities (dmf)."[11] Recent research[12] confirms a plaque-reducing effect and suggests that the compound, having some chemical properties similar to sucrose, attracts and then "starves" harmful micro-organisms, allowing the mouth to remineralise damaged teeth with less interruption. (However, this same effect also interferes with yeast micro-organisms and others, so xylitol is inappropriate for making yeast-based bread, for instance.)
Xylitol based products are allowed by the U.S. Food and Drug Administration to make the medical claim that they do not promote dental cavities.[13]
A recent study demonstrated that a water additive for animals containing xylitol was effective in reducing plaque and calculus accumulation in cats.[14]
Diabetes
Possessing approximately 40% less food energy,[15] xylitol is a low-calorie alternative to table sugar. Absorbed more slowly than sugar, it doesn't contribute to high blood sugar levels or the resulting hyperglycemia caused by insufficient insulin response.
Osteoporosis
Xylitol also appears to have potential as a treatment for osteoporosis. A group of Finnish researchers has found that dietary xylitol prevents weakening of bones in laboratory rats, and actually improves bone density.[16][17]
Ear and upper respiratory infections
Studies have shown that xylitol chewing gum can help prevent ear infections[18] (acute otitis media); the act of chewing and swallowing assists with the disposal of earwax and clearing the middle ear, whilst the presence of xylitol prevents the growth of bacteria in the eustachian tubes (auditory tubes or pharyngotympanic tubes) which connect the nose and ear.[19] When bacteria enter the body they hold on to the tissues by hanging on to a variety of sugar complexes. The open nature of xylitol and its ability to form many different sugar-like structures appears to interfere with the ability of many bacteria to adhere.[20] Xylitol can be applied nasally through a saline solution containing xylitol.
When applied nasally to 21 subjects in a double-blind randomized controlled trial, it significantly reduced the number of nasal coagulase-negative Staphylococcus bacteria compared to the saline control. The researchers believe that it increases the effectiveness of endogenous (naturally present in the body) antimicrobial factors.[21]
Infection
In rats, xylitol has been found to increase the activity of neutrophils, the white blood cells involved in fighting many bacteria. This effect seems to be quite broad, acting even in cases such as general sepsis. [22]
Candida yeast
A recent report suggests that consumption of xylitol may help control oral infections of Candida yeast; in contrast, galactose, glucose, and sucrose may increase proliferation.[23]
Benefits for pregnant or nursing women
Xylitol is not only safe for pregnant and nursing women, but studies show that regular use significantly reduces the probability of transmitting the Streptococcus mutans bacteria, which is responsible for tooth decay, from mother to child during the first two years of life by as much as 80%.[24]
Safety
Xylitol has no known toxicity in humans, and people have consumed as much as 400 grams daily for long periods with no apparent ill effects.[25] Like most sugar alcohols, it has a laxative effect because sugar alcohols are not fully broken down during digestion; albeit ten times weaker than sorbitol. The effect depends upon the individual. In one study of 13 children, 4 experienced diarrhea when consuming over 65 grams per day.[26] Studies have reported that adaptation occurs after several weeks of consumption.[26]
Dogs that have ingested foods containing high levels of xylitol (greater than 100 milligram of xylitol consumed per kilogram of bodyweight) have presented with low blood sugar (hypoglycaemia) which can be life-threatening.[27] Low blood sugar can become manifest as a loss of coordination, depression, collapse and seizures as soon as 30 minutes after ingestion.[28][29] Intake of very high doses of xylitol (greater than 500 - 1000 mg/kg bwt) has also been implicated in liver failure in dogs, which can be fatal.[30] These are points of controversy, however, as earlier World Health Organization studies using much higher doses on dogs for long periods showed no ill effect. [31]
See also
- Other sugar alcohols: mannitol, sorbitol, erythritol, maltitol, lactitol
- Herbal sweetener: stevia
- Artificial sweeteners: aspartame, sucralose
- L-xylulose reductase
References
- ^ MSDS for xylitol
- ^ Gare, Fran (February 1, 2003). The Sweet Miracle of Xylitol. Basic Health Publications, Inc. ISBN 1-59120-038-5.
- ^ R Sreenivas Rao, Ch. Pavanajyothi, RS Prakasham, PN Sharma, L Venkateswar Rao (2006) Xylitol production from corn fibre and sugarcane bagasse hydrolysates by Candida tropicalis Bioresource Technology 97:1974-1978.
- ^ http://www.whatsnewiningredients.com/Food-Ingredients/Articles.aspx/18767
- ^ http://www.ap-foodtechnology.com/Formulation/Danisco-ramps-up-xylitol-production-in-China-new-deal
- ^ Sugar Substitutes: Are They Safe? - HealtHints Newsletter
- ^ Advanced food development and functional foods from Finland
- ^ "Acid production from Lycasin, maltitol, sorbitol and xylitol by oral streptococci and lactobacilli", Acta Odontol Scand 1977; 35: 257–263
{{citation}}: Cite uses deprecated parameter|authors=(help) - ^ "Comparative effects of the substance-sweeteners glucose, sorbitol, sucrose, xylitol and trichlorosucrose on lowering of pH by two oral Streptococcus mutans strains in vitro.", Arch Oral Biol 1980; 24: 965–970
{{citation}}: Cite uses deprecated parameter|authors=(help) - ^ "Xylitol and caries prevention--is it a magic bullet?", British Dental Journal (2003) Apr 26;194(8):429-36
{{citation}}: Cite uses deprecated parameter|authors=(help) - ^ a b American Academy of Pediatric Dentistry. (2006) Policy on the Use of Xylitol in Caries Prevention.
- ^ Tanzer, JM (1995). Xylitol chewing gum and dental caries. International dental journal 45 (1 Suppl 1):65-76. (online abstract)
- ^ U.S. FDA 21 CFR §101.80
- ^ Clarke, D.E. (2006) Drinking Water Additive Decreases Plaque and Calculus Accumulation in Cats. J Vet Dent(23)2:79-82
- ^ www.diabetes.org.nz/food/artificialsweeteners.html
- ^ Mattila PT, Svanberg MJ, Jämsä T, Knuuttila ML (2002). Improved bone biomechanical properties in xylitol-fed aged rats.Metabolism 51(1):92-6. (online abstract)
- ^ Mattila, PT (1999). Dietary xylitol in the prevention of experimental osteoporosis: Beneficial effects on bone resorption, structure and biomechanics. Dissertation, Institute of Dentistry, University of Oulu. (online)
- ^ Uhari M, et al. (1998). A novel use of xylitol sugar in preventing acute otitis media. Pediatrics, 102(4): 879–974.
- ^ Drgreene.com commercial site
- ^ Besttreaments.co.uk
- ^ Zabner J, Seiler MP, Launspach JL; et al. (2000). "The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing". Proceedings of the National Academy of Sciences of the United States of America. 97 (21): 11614–9. doi:10.1073/pnas.97.21.11614. PMC 17249. PMID 11027360.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Renko, Marjo (2008 March 11;8:45.). "Xylitol-supplemented nutrition enhances bacterial killing and prolongs survival of rats in experimental pneumococcal sepsis". BMC Microbiology. 8: 45. doi:10.1186/1471-2180-8-45. PMID 18334022. Retrieved 2008-08-23.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Abu-Elteen, Khaled H. The influence of dietary carbohydrates on in vitro adherence of four Candida species to human buccal epithelial cells. Microbial Ecology in Health and Disease (2005), 17(3), 156-162
- ^ Maternal Xylitol Consumption to Prevent Mother-Child Transmission of Mutans Streptococci
- ^ Mäkinen KK (1976). "Long-term tolerance of healthy human subjects to high amounts of xylitol and fructose: general and biochemical findings". Int Z Vitam Ernahrungsforsch Beih. 15: 92–104. PMID 783060.
- ^ a b Wang YM, van Eys J (1981). "Nutritional significance of fructose and sugar alcohols". Annu. Rev. Nutr. 1: 437–75. doi:10.1146/annurev.nu.01.070181.002253. PMID 6821187.
- ^ Dunayer, E.K., Gwaltney-Brant, S.M. (2006) Acute hepatic failure and coagulopathy associated with xylitol ingestion in dogs, Journal of the American Veterinary Medical Association (229)7:1113-1117
- ^ ASPCA article
- ^ Dunayer, E.K (2004) Hypoglycemia following canine ingestion of xylitol-containing gum, Veterinary and Human Toxicology 46(2):87-88
- ^ Dunayer, E.K (2006) New findings on the effects of xylitol ingestion in dogs Veterinary Medicine 101(12):791-797
- ^ [1] Xlear, Inc. Issues Response to JAVMA Report on Dogs and Xylitol
