Opisthorchis viverrini
| Opisthorchis viverrini | |
|---|---|
| File:Opisthorchis viverrini.jpg | |
| An adult Opisthorchis viverrini prepared on a microscope slide | |
| Scientific classification | |
| Missing taxonomy template (fix): | Opisthorchis viverrini |
| Binomial name | |
| Opisthorchis viverrini | |
| Synonyms[1] | |
|
Distoma viverrini Poirier, 1886 | |
Opisthorchis viverrini, common name Southeast Asian liver fluke, is a trematode parasite from the family Opisthorchiidae that attacks the area of the bile duct. Infection is acquired when people ingest raw or undercooked fish.[2] It causes the disease opisthorchiasis (also called clonorchiasis).[3] Opisthorchis viverrini infection also predisposes the infected for cholangiocarcinoma, a cancer of the gall bladder and/or its ducts.
Opisthorchis viverrini (together with Clonorchis sinensis and Opisthorchis felineus) is one of the three most medically important species in the family Opisthorchiidae.[3]
Opisthorchis viverrini is endemic throughout Thailand, the Lao People's Democratic Republic, Vietnam and Cambodia.[4] In Northern Thailand, it is widely distributed, with high prevalence in humans, while in Central Thailand there is low rate of prevalence.[5] The disease opisthorchiasis (caused by Opisthorchis viverrini) does not occur in southern Thailand.[5]
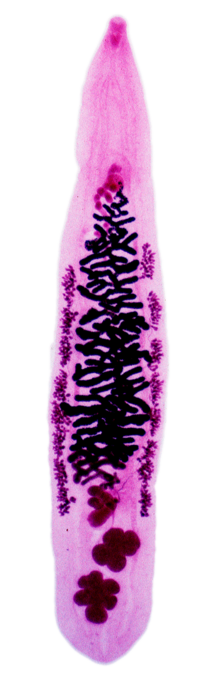
Description

The testes of an adult Opisthorchis viverrini are lobed[1] in comparison of dendritic testes of Clonorchis sinensis.[1]
The eggs of Opisthorchis viverrini are 30 × 12 μm in size[1] and they are slightly narrower and more regularly ovoid than in Clonorchis sinensis.[1] But eggs of Opisthorchis viverrini are visually indisgushiable in Kato technique smears from other eggs of flukes from other fluke family Heterophyidae.[6]
The metacercariae of Opisthorchis viverrini are brownish, eliptical with two nearly equal-sized suckers: the oral sucker and the ventral sucker.[6] They are 0.19-0.25 × 0.15-0.22 mm in size.[6]
Life cycle

Opisthorchis viverrini is a hermaphroditic liver fluke.[4] Its life cycle is similar to the life cycle of Clonorchis sinensis.[4] It involves a freshwater snail, in which asexual reproduction takes place, and freshwater cyprinid fishes (family Cyprinidae) as intermediate hosts. Fish–eating (= piscivorous) mammals, including humans, dogs and cats, act as definitive hosts, in which sexual reproduction occurs.[4] As a result of poor sanitation practices and inadequate sewerage infrastructure, Opisthorchis viverrini-infected people pass the trematode's eggs in their feces into bodies of fresh water.[2]
First intermediate host
The first intermediate hosts include freshwater snails of the genus Bithynia.[7] The only known host is Bithynia siamensis (that include all its three subspecies).[1][8] Aquatic snails, which represent the first intermediate hosts of Opisthorchis viverrini, ingest the eggs from which the miracidia undergo asexual reproduction before a population of the free swimming larval stage, called a cercaria, is shed from the infected snails.[2]
Second intermediate host
The cercaria then locates a cyprinoid fish, encysts in the fins, skin and musculature of the fish, and becomes a metacercaria.[2] Habitats of second intermediate hosts of Opisthorchis viverrini include freshwater habitats with stagnant or slow-moving waters (ponds, river, aquaculture, swamps, rice fields).[9]
In 1965 there were known 9 fish hosts of Opisthorchis viverrini.[10] Up to 2002 there were known 15 species of fishes from 7 genera of the family Cyprinidae, that serves as second intermediate host.[1] Further research by Rim et al. (2008)[6] showed additional five more host species:
- Puntius brevis[6]
- Puntius gonionotus - synonym: Barbonymus gonionotus, Java Barb[6]
- Puntius orphoides[1][6]
- Puntius proctozysron - synonym: Puntioplites proctozystron[6]
- Puntius viehoeveri - synonym: Barbonymus gonionotus[6]
- Hampala dispar[1][6]
- Hampala macrolepidota[6]
- Cyclocheilichthys armatus[6] - synonym: Cyclocheilichthys siaja[11]
- Cyclocheilichthys repasson[6]
- Labiobarbus lineatus[6]
- Esomus metallicus[6]
- Mystacoleucus marginatus[6]
- Puntioplites falcifer[6]
- Onychostoma elongatum[6]
- Osteochilus hasseltii[6]
- Hypsibarbus lagleri[6]
- Barbodes gonionotus - synonym: Barbonymus gonionotus[6]
Definitive host

The metacercarial stage is infective to humans and other fish-eating mammals[2] including dogs and cats.[4] The natural definitive host is the Leopard Cat (Prionailurus bengalensis).[1] The young adult worm escapes from the metacercarial cyst in the upper small intestine and then migrates through the ampulla of Vater into the biliary tree, where it develops to sexual maturity over four to six weeks, thus completing the life cycle.[2]
Fish contain more metacercaria from September to February, before the dry season[1][10] and this is the period, when humans are usually infected.[1] Infection is acquired when people ingest raw or undercooked fish.[2] Dishes of raw fish are common in the cuisine of Laos and the cuisine of Thailand: koi-pla, raw fish in spicy salad lab-pla,[8] salted semi-fermented fish dishes called pla-ra (pla ra),[1] pla-som[8] and Som fak.[6]
The adult worms, which are hermaphrodites, can live for many years in the liver, even decades, shedding as many as 200 eggs per day which pass out via bile into the chyme and feces.[2] The lifespan of Opisthorchis viverrini is over 10 years.[11]
Opisthorchis viverrini secretes a granulin-like growth protein especially in its gut and integument.[13]
Effect on human health
Medical care and loss of wages caused by Opisthorchis viverrini in Laos and in Thailand costs about $120 million annually[1] or $120 million per year can cost Northeast Thailand only.[3]
Infection of Opisthorchis viverrini and of other liver flukes in Asia affect the poor and poorest people.[14] Opisthorchiasis have received less attention in comparison of other diseases and it is a neglected disease in Asia.[14]
Prevalence

19.3% in North Thailand,
15.7% in North East Thailand,
3.8% in Central Thailand,
0% in Southern Thailand.[12]
Opisthorchiasis is prevalent in geographical regions where raw cyprinid fishes are a staple of the diet of humans.[4] The prevalence of human infection can be as high as 70% in some regions, for example in Khon Kaen Province in Thailand.[4] The parasite establishes in the bile ducts of the liver as well as extrahepatic ducts and the gall bladder of the mammalian (definitive) host.[4]
Children under the age of 5 are rarely infected by Opisthorchis viverrini.[1]
In the Lao People's Democratic Republic, the prevalence of opisthorchiasis was:
- 40% in 1992 causing about 1 744 000 people infected[8]
In Thailand, the prevalence of opisthorchiasis was:
- scaterred reports by Verdun & Bruyant (1908), Leiper (1911), Prommas (1927), Bedier & Chesneau (1929)[10]
- The national control programme have started in Thailand in 1950.[15]
- 25% in 1953 causing about 2 million infected people.[8] The first widespread report of opisthorchiasis in Thailand was in 1953.[8]
- 1965: over 3.5 millions infected people[10]
- 14% in 1980-1981 causing about 7 million infected people.[11]
- 63.6% in 1984-1987,[5][15] but another WHO report mention prevalence 35% in Nort-east Thailand for 1984.[8]
- 35.6% in 1988[5]
- 30% in 1989[15]
- The decline of opisthorchiasis was caused by opisthorchiasis control programme, that includes health education including mass distribution of cooking pots[8] and using praziquantel, that was available since 1984.[8]
- 15.2% in 1991 causing 7 million infected people.[8] About 45 million people were at risk of infection.[8] There was prevalence 22.8% in North Thailand, 24.0% in North East Thailand, 7.3% in Central Thailand and 0.3% in Southern Thailand.[8]
- 12% in 1996[15]
- In 1992-1996 the National Public Health Development Plan used the strategy by the Faculty of Tropical Medicine, Mahidol University against opisthorchiasis.[8]
- 7% in 2000[15]
- 9.4% in 2001[5] In Thailand, the prevalence of opisthorchiasis is 9.4 % in 2001,[5] causing about 6 million people are infected with Opisthorchis viverrini.[2][12]
Opisthorchis viverrini was thought to be the only species of liver fluke in Thailand,[11] but PCR techniques have revealed also Clonorchis sinensis in (central) Thailand in 2008.[16]
Another reference from 2002 lists worldwide number of cases about 9 million (without year of estimation).[1] In Thailand, about 7.3 million.[1] About 50 million people are at risk of infection.[1]
In 2005, 67.3 million of people worldwide are at risk of infection.[9]
Keiser & Utzinger (2005)[9] have speculated that aquaculture development is the key risk factor for foodborne trematodiases including opisthorchiasis caused by Opisthorchis viverrini.[9]
Symptoms

Symptoms of opisthorchiasis (caused by Opisthorchis spp.) are indistinguishable from clonorchiasis (caused by Clonorchis sinensis),[3] so the disease should be referred as clonorchiasis.[3]
About 80% of infected people have no symptoms, though they can have eosinophilia.[1] This is when the infection is weak and there are less than 1000 eggs in one gram in feces.[1]
When there are 10.000-30.000 eggs in one gram of feces, then the infection is heavy.[1] Symptoms of heavier infections with Opisthorchis viverrini may include: diarrhoea, pain in epigastric and pain in the upper right quadrant, lack of appetite (anorexia), fatigue, yellowing of the eyes and skin (jaundice) and mild fever.[1]
These parasites are long-lived and cause heavy chronic infections may led to accumulation of fluid in legs (edema) and in the peritoneal cavity (ascites),[1] enlarged non-functional gall-bladder[1] and also cholangitis,[4] which can lead to periductal fibrosis, cholecystitis and cholelithiasis, obstructive jaundice, hepatomegaly and/or fibrosis of the periportal system.[4]
Importantly, both experimental and epidemiological evidence strongly implicates Opisthorchis viverrini infections in the etiology of a malignant cancer of the bile ducts (cholangiocarcinoma) in humans which has a very poor prognosis.[4] Indeed, Clonorchis sinensis and Opisthorchis viverrini are both categorized by the International Agency for Research on Cancer (IARC) as Group 1 carcinogens.[4]
In humans, the onset of cholangiocarcinoma occurs with chronic opisthorchiasis, associated with hepatobiliary damage, inflammation, periductal fibrosis and/or cellular responses to antigens from the infecting fluke.[4] These conditions predispose to cholangiocarcinoma, possibly through an enhanced susceptibility of DNA to damage by carcinogens.[4] Chronic hepatobiliary damage is reported to be multi-factorial and considered to arise from a continued mechanical irritation of the epithelium by the flukes present, particularly via their suckers, metabolites and excreted/secreted antigens as well as immunopathological processes.[4] In regions where Opisthorchis viverrini is highly endemic, the incidence of cholangiocarcinoma is unprecedented.[4] For instance, cholangiocarcinomas represent 15% of primary liver cancer worldwide, but in Thailand's Khon Kaen region, this figure escalates to 90%, the highest recorded incidence of this cancer in the world.[4] Of all cancers worldwide from 2002, 0.02% were cholangiocarcinoma caused by Opisthorchis viverrini.[12]
The cancer of the bile ducts caused by opisthorchiasis occur in the ages 25–44 years in Thailand.[8]
Diagnosis
For medical diagnosis there is need to find eggs of Opisthorchis viverrini in feces[1] using Kato technique.[8]
An antigen 89 kDa of Opisthorchis viverrini can be detected by ELISA test.[1]
A PCR test capable of amplifying a segment of the internal transcribed spacer region of ribosomal DNA for the opisthorchiid and heterophyid flukes eggs taken directly from faeces was developed and evaluated in a rural community in central Thailand.[16] The lowest quantity of DNA that could be amplified from individual adults of Opisthorchis viverrini was estimated to 0.6 pg.[16]
Prevention
Currently, there is no effective chemotherapy to combat cholangiocarcinoma, such that intervention strategies need to rely on the prevention or treatment of liver fluke infection/disease.[4] Although effective prevention could be readily achieved by persuading people to consume cooked fish (via education programs), the ancient cultural custom to consume raw, undercooked or freshly pickled fish persists in endemic areas.[4] Cooking or deep-freezing (-20 °C for 7 days)[15] of food made of fish is sure method of prevention.[1] Methods for prevention of Opisthorchis viverrini in aquaculture fish ponds were proposed by Khamboonruang et al. (1997).[17]
Treatment
There was unsuccessful use of chloroquine for opisthorchiasis treatment in 1951-1968.[8]
Thus, currently, the control of opisthorchiasis relies predominantly on anthelmintic treatment with praziquantel.[4] The single dose of praziquantel of 40 mg/kg is effective against opisthorchiasis and also against schistosomiasis.[8] A randomised-controlled trial published in 2011 showed that the broad-spectrum anti-helminthic, tribendimidine, appears to be at least as efficacious as praziquantel.[18]
Artemisinin was also found to have anthelmintic activity against Opisthorchis viverrini.[19]
Despite the efficacy of this compound, the lack of an acquired immunity to infection predisposes humans to reinfections in endemic regions.[4] In addition, under experimental conditions, the short-term treatment of Opisthorchis viverrini-infected hamsters with praziquantel (400 mg per kg of live weight) has been shown to induce a dispersion of parasite antigens, resulting in adverse immunopathological changes as a result of oxidative and nitrative stresses following re-infection with Opisthorchis viverrini, a process which has been proposed to initiate and/or promote the development of cholangiocarcinoma in humans.[4]
Given the current reliance on a single trematocidal drug against Opisthorchis viverrini, there is substantial merit in searching for new intervention methods, built on a detailed understanding of the interplay between the parasites and their hosts as well as the biology of the parasites themselves at the molecular level.[4] Furthermore, the characterization of the genes expressed in these parasites should assist in elucidating the molecular mechanisms by which opisthorchiasis initiate and enhance the development of cholangiocarcinoma.[4]
Genetics
Currently, a total of only ~5,000[2] expressed sequence tags (ESTs) are publicly available for Opisthorchis viverrini, a dataset far too small to give sufficient insights into transcriptomes for the purpose of supporting genomic and other fundamental molecular research.[4]
Although the genome size of Opisthorchis viverrini has not yet been reported, it is known to have six pairs of chromosomes, i.e. 2n = 12.[2]
References
This article incorporates CC-BY-2.5 text from references [4][12][16] and CC-BY-2.0 text from the reference.[2]
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa Muller R. & Wakelin D. (2002). Worms and human disease. CABI. page 43-44.
- ^ a b c d e f g h i j k l Laha T., Pinlaor P., Mulvenna J., Sripa B., Sripa M., et al. (2007). "Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics 8: 189. doi:10.1186/1471-2164-8-189.
- ^ a b c d e King1 S. & Scholz T. (2001). "Trematodes of the family Opisthorchiidae: a minireview". Korean Journal of Parasitology 39(3): 209–221. doi:10.3347/kjp.2001.39.3.209, PMC PMC2721069.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z Young N.D., Campbell B.E., Hall R.S., Jex A.R., Cantacessi C., et al. (2010). "Unlocking the Transcriptomes of Two Carcinogenic Parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Neglected Tropical Diseases 4(6): e719. doi:10.1371/journal.pntd.0000719.
- ^ a b c d e f Jongsuksuntigul P. & Imsomboon T. (2003). "Opisthorchiasis control in Thailand". Acta Tropica 88(3): 229–232. doi:10.1016/j.actatropica.2003.01.002.
- ^ a b c d e f g h i j k l m n o p q r s t u v Rim H.-J., Sohn W.-M., Yong T.-S., Eom K. S., Chai J.-Y., Min D.-Y., Lee S.-H., Hoang E.-H., Phommasack B. & Insisengmay S. (2008). "Fishborne Trematode Metacercariae Detected in Freshwater Fish from Vientiane Municipality and Savannakhet Province, Lao PDR". Korean Journal of Parasitology 46(4): 253–260. PMC PMC2612611, doi:10.3347/kjp.2008.46.4.253.
- ^ Tohamy A. A. & Mohamed S. M. (2006). "Chromosomal studies on two Egyptian freshwater snails, Cleopatra and Bithynia (Mollusca-Prosobranchiata)". Arab J. Biotech. 9(1): 17-26. PDF.
- ^ a b c d e f g h i j k l m n o p q World Health Organization (1995). Control of Foodborne Trematode Infection. WHO Technical Report Series. 849. PDF part 1, PDF part 2. page 89-91.
- ^ a b c d Keiser J. & Utzinger J. (2005). "Emerging foodborne trematodiasis". Emerging Infectious Diseases [serial on the Internet]. 2005 Oct. Available from http://www.cdc.gov/ncidod/EID/vol11no10/05-0614.htm
- ^ a b c d Wykoff D.E., Harinasuta C., Juttijudata P. & Winn M.M. (1965). "Opisthorchis viverrini in Thailand: The Life Cycle and Comparison with O. felineus". The Journal of Parasitology 51(2): 207-214. PMID 14275209, JSTOR.
- ^ a b c d Harinasuta C. & Harinasuta T. (1984). "Opisthorchis viverrini: life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand". Arzneimittelforschung 34(9B): 1164-1167. PMID 6542383
- ^ a b c d e Sripa B., Kaewkes S., Sithithaworn P., Mairiang E., Laha T., et al. (2007). "Liver Fluke Induces Cholangiocarcinoma". PLoS Medicine 4(7): e201. doi:10.1371/journal.pmed.0040201.
- ^ Smout M.J., Laha T., Mulvenna J., Sripa B., Suttiprapa S. et al. (2009). "A Granulin-Like Growth Factor Secreted by the Carcinogenic Liver Fluke, Opisthorchis viverrini, Promotes Proliferation of Host Cells". PLoS Pathogens 5(10): e1000611. doi:10.1371/journal.ppat.1000611.
- ^ a b Sripa B. (2008) "Concerted Action Is Needed to Tackle Liver Fluke Infections in Asia". PLoS Neglected Tropical Diseases 2(5): e232. doi:10.1371/journal.pntd.0000232.
- ^ a b c d e f World Health Organization (2004). REPORT JOINT WHO/FAO WORKSHOP ON FOOD-BORNE TREMATODE INFECTIONS IN ASIA. Report series number: RS/2002/GE/40(VTN). 55 pp. PDF. pages 15-17.
- ^ a b c d Traub R.J., Macaranas J., Mungthin M., Leelayoova S., Cribb T., et al. (2009). "A New PCR-Based Approach Indicates the Range of Clonorchis sinensis Now Extends to Central Thailand". PLoS Neglected Tropical Diseases 3(1): e367. doi:10.1371/journal.pntd.0000367.
- ^ Khamboonruang C., Keawvichit R., Wongworapat K., Suwanrangsi S., Hongpromyart M., Sukhawat K., Tonguthai K. & Lima dos Santos C. A. (1997). "Application of hazard analysis critical control point (HACCP) as a possible control measure for Opisthorchis viverrini infection in cultured carp (Puntius gonionotus)". The Southeast Asian journal of tropical medicine and public health 28(Suppl 1): 65-72. PMID 9656352.
- ^ Soukhathammavong P, Odermatt P, Sayasone S; et al. (2011). "Efficacy and safety of mefloquine, artesunate, mefloquine—artesunate, tribendimidine, and praziquantel in patients with Opisthorchis viverrini: a randomised, exploratory, open-label, phase 2 trial". Lancet Infect Dis. 11 (2): 110–118. doi:10.1016/S1473-3099(10)70250-4.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Keiser J. & Utzinger J. (2007). "Artemisinins and synthetic trioxolanes in the treatment of helminth infections". Current opinion in infectious diseases 20(6):605-612. PMID 17975411.
External links
- "Opisthorchiasis — Opisthorchis felineus Opisthorchis viverrini". DPDx Laboratory Identification of Parasites of Public Health Concern. Centers for Disease Control and Prevention.
- "Introduction — Opisthorchiasis". Parasites and Pestilence. The Program in Human Biology, Stanford University.
- Riganti M, Pungpak S, Punpoowong B, Bunnag D, Harinasuta T (1989). "Human pathology of Opisthorchis viverrini infection: a comparison of adults and children". Southeast Asian J. Trop. Med. Public Health. 20 (1): 95–100. PMID 2772709.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Upatham E.S. & Viyanant V. (2003). "Opisthorchis viverrini and opisthorchiasis: a historical review and future perspective". Acta Tropica 88(3): 171–176. doi:10.1016/j.actatropica.2003.01.001.
- Andrews R. H., Sithithaworn P. & Petney T. N. (2008). "Opisthorchis viverrini: an underestimated parasite in world health". Trends Parasitol. 24(11): 497–501. doi:10.1016/j.pt.2008.08.011, PMC PMC2635548
- Kaewpitoon N., Kaewpitoon S. J., Pengsaa P. & Sripa B. (2008). "Opisthorchis viverrini: The carcinogenic human liver fluke". World Journal of Gastroenterology 14(5): 666-674. doi:10.3748/wjg.14.666. HTM PMC PMC2683991.
- Kaewkes S. (2003). "Taxonomy and biology of liver flukes". Acta Tropica 88(3): 177-186. doi:10.1016/j.actatropica.2003.05.001, PMID 14611872
- Adam R., Arnold H., Hinz E. & Storch V. (1995). "Morphology and ultrastructure of the redia and pre-emergent cercaria of Opisthorchis viverrini (Trematoda: Digenea) in the intermediate host Bithynia siamensis goniomphalus (Prosobranchia: Bithyniidae)". Applied parasitology 36(2): 136-154. PMID 7550441
- Inatomi S., Tongu Y., Sakumoto D., Suguri S. & Itano K. (1971). "The ultrastructure of helminth. VI. The body wall of Opisthorchis viverrini (Poirier, 1886)". Acta medica Okayama 25(2): 129-142. PMID 4333630.

