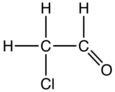
Chloroacetaldehyde
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloroacetaldehyde | |||
| Systematic IUPAC name
Chloroethanal | |||
| Other names
2-Chloroacetaldehyde
2-Chloroethanal | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.158 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H3ClO | |||
| Molar mass | 78.50 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | acrid, penetrating[1] | ||
| Density | 1.117 g/mL | ||
| Melting point | −16.3 °C (2.7 °F; 256.8 K) hydrate melts at 43–50 °C[1] | ||
| Boiling point | 85 to 85.5 °C (185.0 to 185.9 °F; 358.1 to 358.6 K) | ||
| soluble[1] | |||
| Solubility | organic solvents | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
alkylating agent | ||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H301, H311, H314, H330, H351, H400 | |||
| Flash point | 87.7 °C (189.9 °F) (closed cup) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
89 mg/kg (oral, rat) 82 mg/kg (oral, mouse)[3] | ||
LC50 (median concentration)
|
200 ppm (rat, 1 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
C 1 ppm (3 mg/m3)[2] | ||
REL (Recommended)
|
C 1 ppm (3 mg/m3)[2] | ||
IDLH (Immediate danger)
|
45 ppm[2] | ||
| Related compounds | |||
Related compounds
|
2-chloroethanol, Chloroacetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.
Chloroacetaldehyde is a metabolite of the antineoplastic ifosfamide and believed to be responsible for some of the toxicity observed with ifosfamide.
Synthesis and occurrence
[edit]Hydrated chloroacetaldehyde is produced by the chlorination of aqueous vinyl chloride:
- ClCH=CH2 + Cl2 + H2O → ClCH2CHO + 2 HCl
It can also be prepared from vinyl acetate[5] or by careful chlorination of acetaldehyde.[1] The related bromoacetaldehyde is prepared via bromination of vinyl acetate. It also rapidly forms an acetals in the presence of alcohols.[6]
Water free chloroacetaldehyde is prepared from the hydrate by azeotropic distillation with chloroform, toluene, or carbon tetrachloride. Anhydrous chloroacetaldehyde reversibly converts to polyacetals.[7][1] Less reactive chloroacetaldehyde derivatives might be used instead to obtain chloroacetaldehyde or bypass its intermediate formation completely: e.g. chloroacetaldehyde dimethyl acetal (2-chloro-1,1-dimethoxyethane) hydrolyzes in acidic conditions to give chloroacetaldehyde, which may then quickly react with the other reagents[7] instead of polymerizing.
Relevant to its occurrence in humans, it arises via the isomerization of chloroethylene oxide, a metabolite of vinyl chloride.[8]
Reactions
[edit]Chloroacetaldehyde readily hydrates:
Being bifunctional, chloroacetaldehyde is a precursor to many heterocyclic compounds. It condenses with thiourea derivatives to give aminothiazoles. This reaction was once used in the preparation of sulfathiazole, one of the first sulfa drugs.[5] Chloroacetaldehyde is a building block in the synthesis of the pharmaceuticals altizide, polythiazide, brotizolam, and ciclotizolam.[7] Chloroacetaldehyde is an alkylating agent. It reacts with adenosine and cytidine to give cyclic products containing a fused imidazole group. This reaction is related to the possible mutagenic properties of chloroacetaldehyde.[9]
Environmental aspects
[edit]Chloroacetaldehyde is a metabolite in the degradation of 1,2-dichloroethane, which initially converts to chloroethanol. This metabolic pathway is topical because 1,2-dichloroethane is produced on a large scale as a precursor to vinyl chloride.[10]
Safety
[edit]Chloroacetaldehyde is corrosive to mucous membranes. It irritates eyes, skin and respiratory tract.[1]
Based on data collected from human studies in 1962, exposures to 45 ppm of chloroacetaldehyde were found to be disagreeable and caused conjunctival irritation to the subjects.[11] The Occupational Safety and Health Administration established a permissible exposure limit at a ceiling of 1 ppm (3 mg/m3) for exposures to chloroacetaldehyde.[12]
See also
[edit]References
[edit]- ^ a b c d e f The Merck index. S Budavari, M O'Neil, A Smith (12 ed.). Merck. 1996. p. 2108. ISBN 9780911910124.
{{cite book}}: CS1 maint: others (link) - ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0118". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Chloroacetaldehyde". National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 20 February 2015.
- ^ "Chloroacetaldehyde". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Jira, Reinhard; Kopp, Erwin; McKusick, Blaine C.; Röderer, Gerhard; Bosch, Axel; Fleischmann, Gerald (2007). "Chloroacetaldehydes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_527.pub2. ISBN 978-3527306732.
- ^ S. M. McElvain and D. Kundiger "Bromoacetal" Organic Syntheses 1943, volume 23, p. 8. doi:10.15227/orgsyn.023.0008.
- ^ a b c Keiji, T (1992-10-30). "α-Chlorocarbonyl Compounds: Their Synthesis and Applications (Commemoration Issue Dedicated to Professor Shigeo Tanimoto On the Occasion of His Retirement)". Bulletin of the Institute for Chemical Research, Kyoto University. 70 (3): 341. hdl:2433/77455. ISSN 0023-6071.
- ^ Swenberg, J. A.; Lu, K.; Moeller, B. C.; Gao, L.; Upton, P. B.; Nakamura, J.; Starr, T. B. (2011). "Endogenous versus Exogenous DNA Adducts: Their Role in Carcinogenesis, Epidemiology, and Risk Assessment". Toxicological Sciences. 120 (Suppl 1): S130–S145. doi:10.1093/toxsci/kfq371. PMC 3043087. PMID 21163908.
- ^ Singer, B. (1996). "DNA Damage: Chemistry, Repair, and Mutagenic Potential". Regulatory Toxicology and Pharmacology. 23 (1 Pt 1): 2–13. doi:10.1006/rtph.1996.0002. PMID 8628915.
- ^ Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and Biochemistry of 1,2-Dichloroethane Degradation", Biodegradation, 1994, 5, 249-57.doi:10.1007/BF00696463
- ^ Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards