Purkinje cell
| Purkinje cell | |
|---|---|
 Drawing of pigeon Purkinje cells (A) by Santiago Ramon y Cajal | |
| Details | |
| Pronunciation | Often pronounced as /pɜːrˈkɪndʒi/ pur-KIN-jee;[1] but Czech pronunciation is (Czech: [ˈpurkɪɲɛ] cells |
| Location | Cerebellum |
| Shape | flat dendritic arbor |
| Function | inhibitory projection neuron |
| Neurotransmitter | GABA |
| Presynaptic connections | Parallel fibers and Climbing fibers |
| Postsynaptic connections | Cerebellar deep nuclei |
| Identifiers | |
| MeSH | D011689 |
| NeuroNames | 365 |
| NeuroLex ID | sao471801888 |
| TA98 | A14.1.07.404 |
| FMA | 67969 |
| Anatomical terms of neuroanatomy | |
Purkinje cells, or Purkinje neurons, are a class of GABAergic inhibitory neurons located in the cerebellum.[2] They are named after their discoverer, Czech anatomist Jan Evangelista Purkyně, who characterized the cells in 1839.
Structure





These cells are some of the largest neurons in the human brain (Betz cells being the largest),[3] with an intricately elaborate dendritic arbor, characterized by a large number of dendritic spines. Purkinje cells are found within the Purkinje layer in the cerebellum. Purkinje cells are aligned like dominos stacked one in front of the other. Their large dendritic arbors form nearly two-dimensional layers through which parallel fibers from the deeper-layers pass. These parallel fibers make relatively weaker excitatory (glutamatergic) synapses to spines in the Purkinje cell dendrite, whereas climbing fibers originating from the inferior olivary nucleus in the medulla provide very powerful excitatory input to the proximal dendrites and cell soma. Parallel fibers pass orthogonally through the Purkinje neuron's dendritic arbor, with up to 200,000 parallel fibers[4] forming a Granule-cell-Purkinje-cell synapse with a single Purkinje cell. Each Purkinje cell receives approximately 500 climbing fiber synapses, all originating from a single climbing fiber.[5] Both basket and stellate cells (found in the cerebellar molecular layer) provide inhibitory (GABAergic) input to the Purkinje cell, with basket cells synapsing on the Purkinje cell axon initial segment and stellate cells onto the dendrites.
Purkinje cells send inhibitory projections to the deep cerebellar nuclei, and constitute the sole output of all motor coordination in the cerebellar cortex.
Molecular
The Purkinje layer of the cerebellum, which contains the cell bodies of the Purkinje cells and Bergmann glia, express a large number of unique genes.[6] Purkinje-specific gene markers were also proposed by comparing the transcriptome of Purkinje-deficient mice with that of wild-type mice.[7] One illustrative example is the Purkinje cell protein 4 (PCP4) in knockout mice, which exhibit impaired locomotor learning and markedly altered synaptic plasticity in Purkinje neurons.[8][9] PCP4 accelerates both the association and dissociation of calcium (Ca2+) with calmodulin (CaM) in the cytoplasm of Purkinje cells, and its absence impairs the physiology of these neurons.[8][9][10][11]
Development
Mammalian embryonic research has detailed the neurogenic origins of Purkinje cells.[12] During early development Purkinje cells arise in the ventricular zone in the neural tube, the nervous system´s precursor in the embryo. All cerebellar neurons derive from germinal neuroepithelia from the ventricular zone.[13] Purkinje cells are specifically generated from progenitors in the ventricular neuroepithelium of the embryonic cerebellar primordium.[14] The first cells generated from the cerebellar primordium form a cap over a diamond-shaped cavity of the developing brain called the fourth ventricle forming the two cerebellar hemispheres. The Purkinje cells that develop later are those of the cerebellum's center-lying section called the vermis. They develop in the cerebellar primordium that covers the fourth ventricle and below a fissure-like region called the isthmus of the developing brain. Purkinje cells migrate toward the outer surface of the cerebellar cortex and form the Purkinje cell layer.
Purkinje cells are born during the earliest stages of cerebellar neurogenesis. Neurogenin2, together with neurogenin1, are transiently expressed in restricted domains of the ventricular neuroepithelium during the time-window of Purkinje cell genesis.[15] This spatio-temporal distribution pattern suggests that neurogenins are involved in the specification of phenotypically heterogeneous Purkinje cell subsets, ultimately responsible for constructing the framework of the cerebellar topography.
There is evidence in mice and humans that bone marrow cells either fuse with or generate cerebellar Purkinje cells, and it is possible that bone marrow cells, either by direct generation or by cell fusion, could play a role in repair of central nervous system damage.[16][17][18][19][20] Further evidence points yet towards the possibility of a common stem cell ancestor among Purkinje neurons, B-lymphocytes and aldosterone-producing cells of the human adrenal cortex.[19]
Function
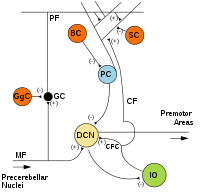
MF: Mossy fiber.
DCN: Deep cerebellar nuclei.
IO: Inferior olive.
CF: Climbing fiber.
GC: Granule cell.
PF: Parallel fiber.
PC: Purkinje cell.
GgC: Golgi cell.
SC: Stellate cell.
BC: Basket cell.
Purkinje cells show two distinct forms of electrophysiological activity:
- Simple spikes occur at rates of 17 – 150 Hz (Raman and Bean, 1999), either spontaneously or when Purkinje cells are activated synaptically by the parallel fibers, the axons of the granule cells.
- Complex spikes are slow, 1–3 Hz spikes, characterized by an initial prolonged large-amplitude spike, followed by a high-frequency burst of smaller-amplitude action potentials. They are caused by climbing fiber activation and can involve the generation of calcium-mediated action potentials in the dendrites. Following complex spike activity, simple spikes can be suppressed by the powerful complex spike input.[21]
Purkinje cells show spontaneous electrophysiological activity in the form of trains of spikes both sodium-dependent and calcium-dependent. This was initially shown by Rodolfo Llinas (Llinas and Hess (1977) and Llinas and Sugimori (1980)). P-type calcium channels were named after Purkinje cells, where they were initially encountered (Llinas et al. 1989), which are crucial in cerebellar function. Activation of the Purkinje cell by climbing fibers can shift its activity from a quiet state to a spontaneously active state and vice versa, serving as a kind of toggle switch.[22] These findings have been challenged by a study suggesting that such toggling by climbing-fiber inputs occurs predominantly in anaesthetized animals and that Purkinje cells in awake behaving animals, in general, operate almost continuously in the upstate.[23] But this latter study has itself been challenged[24] and Purkinje cell toggling has since been observed in awake cats.[25] A computational model of the Purkinje cell has shown intracellular calcium computations to be responsible for toggling.[26]
Findings have suggested that Purkinje cell dendrites release endocannabinoids that can transiently downregulate both excitatory and inhibitory synapses.[27] The intrinsic activity mode of Purkinje cells is set and controlled by the sodium-potassium pump.[28] This suggests that the pump might not be simply a homeostatic, "housekeeping" molecule for ionic gradients. Instead, it could be a computation element in the cerebellum and the brain.[29] Indeed, a mutation in the Na+
-K+
pump causes rapid onset dystonia parkinsonism; its symptoms indicate that it is a pathology of cerebellar computation.[30]
Furthermore, using the poison ouabain to block Na+
-K+
pumps in the cerebellum of a live mouse induces ataxia and dystonia.[31] Numerical modeling of experimental data suggests that, in vivo, the Na+
-K+
pump produces long quiescent punctuations (>> 1 s) to Purkinje neuron firing; these may have a computational role.[32] Alcohol inhibits Na+
-K+
pumps in the cerebellum and this is likely how it corrupts cerebellar computation and body co-ordination.[33][34]
Clinical significance
In humans, Purkinje cells can be harmed by a variety causes: toxic exposure, e.g. to alcohol or lithium; autoimmune diseases; genetic mutations causing spinocerebellar ataxias, gluten ataxia, Unverricht-Lundborg disease, or autism; and neurodegenerative diseases that are not known to have a genetic basis, such as the cerebellar type of multiple system atrophy or sporadic ataxias.[35][36]
Gluten ataxia is an autoimmune disease triggered by the ingestion of gluten.[37] The death of Purkinje cells as a result of gluten exposure is irreversible. Early diagnosis and treatment with a gluten-free diet can improve ataxia and prevent its progression.[35][38] Less than 10% of people with gluten ataxia present any gastrointestinal symptom, yet about 40% have intestinal damage.[38] It accounts for 40% of ataxias of unknown origin and 15% of all ataxias.[38]
The neurodegenerative disease spinocerebellar ataxia type 1 (SCA1) is caused by an unstable polyglutamine expansion within the Ataxin 1 protein. This defect in Ataxin 1 protein causes impairment of mitochondria in Purkinje cells, leading to premature degeneration of the Purkinje cells.[39] As a consequence, motor coordination declines and eventually death ensues.
Some domestic animals can develop a condition where the Purkinje cells begin to atrophy shortly after birth, called cerebellar abiotrophy. It can lead to symptoms such as ataxia, intention tremors, hyperreactivity, lack of menace reflex, stiff or high-stepping gait, apparent lack of awareness of foot position (sometimes standing or walking with a foot knuckled over), and a general inability to determine space and distance.[40] A similar condition known as cerebellar hypoplasia occurs when Purkinje cells fail to develop in utero or die off before birth.
The genetic conditions ataxia telangiectasia and Niemann Pick disease type C, as well as cerebellar essential tremor, involve the progressive loss of Purkinje cells. In Alzheimer's disease, spinal pathology is sometimes seen, as well as loss of dendritic branches of the Purkinje cells.[41] Purkinje cells can also be damaged by the rabies virus as it migrates from the site of infection in the periphery to the central nervous system.[42]
Etymology
Purkinje cells are named after the Czech scientist Jan Evangelista Purkyně, who discovered them in 1839.
References
- ^ Jones, Daniel (2011). Roach, Peter; Setter, Jane; Esling, John (eds.). Cambridge English Pronouncing Dictionary (18th ed.). Cambridge University Press. ISBN 978-0-521-15255-6.
- ^ Komuro, Y.; Kumada, T.; Ohno, N.; Foote, K. D.; Komuro, H. (2013-01-01), Rubenstein, John L. R.; Rakic, Pasko (eds.), "Chapter 15 - Migration in the Cerebellum", Cellular Migration and Formation of Neuronal Connections, Oxford: Academic Press, pp. 281–297, doi:10.1016/b978-0-12-397266-8.00030-2, ISBN 978-0-12-397266-8, retrieved 2020-11-18
- ^ Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A, McNamara JO, and White LE (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 432–4. ISBN 978-0-87893-697-7.
- ^ Tyrrell, T; Willshaw, D (1992-05-29). "Cerebellar cortex: its simulation and the relevance of Marr's theory". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 336 (1277): 239–57. Bibcode:1992RSPTB.336..239T. doi:10.1098/rstb.1992.0059. PMID 1353267.
- ^ Wadiche, JI; Jahr, CE (2001-10-25). "Multivesicular release at climbing fiber-Purkinje cell synapses". Neuron. 32 (2): 301–13. doi:10.1016/S0896-6273(01)00488-3. PMID 11683999.
- ^ Kirsch, L; Liscovitch, N; Chechik, G (December 2012). Ohler, Uwe (ed.). "Localizing Genes to Cerebellar Layers by Classifying ISH Images". PLOS Computational Biology. 8 (12): e1002790. Bibcode:2012PLSCB...8E2790K. doi:10.1371/journal.pcbi.1002790. PMC 3527225. PMID 23284274.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rong, Y; Wang T; Morgan J (2004). "Identification of candidate purkinje cell-specific markers by gene expression profiling in wild-type and pcd3j mice". Molecular Brain Research. 13 (2): 128–145. doi:10.1016/j.molbrainres.2004.10.015. PMID 15582153.
- ^ a b c Felizola SJ, Nakamura Y, Ono Y, Kitamura K, Kikuchi K, Onodera Y, Ise K, Takase K, Sugawara A, Hattangady N, Rainey WE, Satoh F, Sasano H (Apr 2014). "PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues". Journal of Molecular Endocrinology. 52 (2): 159–167. doi:10.1530/JME-13-0248. PMC 4103644. PMID 24403568.
- ^ a b Wei P, Blundon JA, Rong Y, Zakharenko SS, Morgan JI (2011). "Impaired locomotor learning and altered cerebellar synaptic plasticity in pep-19/PCP4-null mice". Mol. Cell. Biol. 31 (14): 2838–44. doi:10.1128/MCB.05208-11. PMC 3133400. PMID 21576365.
- ^ Putkey JA, Kleerekoper Q, Gaertner TR, Waxham MN (2004). "A new role for IQ motif proteins in regulating calmodulin function". J. Biol. Chem. 278 (50): 49667–70. doi:10.1074/jbc.C300372200. PMID 14551202.
- ^ Kleerekoper QK, Putkey JA (2009). "PEP-19, an intrinsically disordered regulator of calmodulin signaling". J. Biol. Chem. 284 (12): 7455–64. doi:10.1074/jbc.M808067200. PMC 2658041. PMID 19106096.
- ^ Sotelo C, Rossi F (2013). "Purkinje Cell Migration and Differentiation". Handbook of the Cerebellum and Cerebellar Disorders: 147–178. doi:10.1007/978-94-007-1333-8_9. ISBN 978-94-007-1332-1. S2CID 80927298.
- ^ Hoshino M (2006). "Molecular machinery governing GABAergic neuron specification in the cerebellum". Cerebellum. 5 (3): 193–198. doi:10.1080/14734220600589202. PMID 16997750. S2CID 20937713.
- ^ Carletti B, Rossi F (2008). "Neurogenesis in the cerebellum". Neuroscientist. 14 (1): 91–100. doi:10.1177/1073858407304629. PMID 17911211. S2CID 34889988.
- ^ Zordan P, Croci L, Hawkes R, Consalez GG (2008). "Comparative analysis of proneural gene expression in the embryonic cerebellum". Dev Dyn. 237 (6): 726–735. doi:10.1002/dvdy.21571. PMID 18498101.
- ^ Hess DC, Hill WD, Carroll JE, Borlongan CV (2004). "Do bone marrow cells generate neurons?". Archives of Neurology. 61 (4): 483–485. doi:10.1001/archneur.61.4.483. PMID 15096394.
- ^ Weimann JM, Johansson CB, Trejo A, Blau HM (2003). "Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant". Nature Cell Biology. 5 (11): 959–966. doi:10.1038/ncb1053. PMID 14562057. S2CID 33685652.
- ^ Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A (2003). "Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes". Nature. 425 (6961): 968–973. Bibcode:2003Natur.425..968A. doi:10.1038/nature02069. hdl:2027.42/62789. PMID 14555960. S2CID 4394453.
- ^ a b Felizola SJ, Katsu K, Ise K, Nakamura Y, Arai Y, Satoh F, Sasano H (2015). "Pre-B lymphocyte protein 3 (VPREB3) expression in the adrenal cortex: precedent for non-immunological roles in normal and neoplastic human tissues". Endocrine Pathology. 26 (2): 119–28. doi:10.1007/s12022-015-9366-7. PMID 25861052. S2CID 27271366.
- ^ Kemp K, Wilkins A, Scolding N (2014). "Cell fusion in the brain: two cells forward, one cell back". Acta Neuropathologica. 128 (5): 629–638. doi:10.1007/s00401-014-1303-1. PMC 4201757. PMID 24899142.
- ^ Eric R. Kandel, James H. Schwartz, Thomas M. Jessell (2000). Principles of Neural Science. 4/e. McGraw-Hill. pp.837-40.
- ^ Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, et al. (2005). "Bistability of cerebellar Purkinje cells modulated by sensory stimulation". Nature Neuroscience. 8 (2): 202–211. doi:10.1038/nn1393. PMID 15665875. S2CID 5543355.
- ^ Schonewille M, Khosrovani S, Winkelman BH, Hoebeek FE, DeJeu MT, Larsen IM, et al. (2006). "Purkinje cells in awake behaving animals operate at the up state membrane potential". Nature Neuroscience. 9 (4): 459–461. doi:10.1038/nn0406-459. PMID 16568098.
- ^ Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, et al. (2006). "Purkinje cells in awake behaving animals operate at the up state membrane potential–Reply". Nature Neuroscience. 9: 461. doi:10.1038/nn0406-461. S2CID 28713325.
- ^ Yartsev MM, Givon-Mayo R, Maller M, Donchin O (2009). "Pausing Purkinje cells in the cerebellum of the awake cat". Frontiers in Systems Neuroscience. 3: 2. doi:10.3389/neuro.06.002.2009. PMC 2671936. PMID 19390639.
- ^ Forrest MD (2014). "Intracellular Calcium Dynamics Permit a Purkinje Neuron Model to Perform Toggle and Gain Computations Upon its Inputs". Frontiers in Computational Neuroscience. 8: 86. doi:10.3389/fncom.2014.00086. PMC 4138505. PMID 25191262.
- ^ Kreitzer AC, Regehr WG (March 2001). "Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells". Neuron. 29 (3): 717–27. doi:10.1016/S0896-6273(01)00246-X. PMID 11301030.
- ^ Forrest MD, Wall MJ, Press DA, Feng J (December 2012). Cymbalyuk G (ed.). "The Sodium-Potassium Pump Controls the Intrinsic Firing of the Cerebellar Purkinje Neuron". PLOS ONE. 7 (12): e51169. Bibcode:2012PLoSO...751169F. doi:10.1371/journal.pone.0051169. PMC 3527461. PMID 23284664.
- ^ Forrest MD (December 2014). "The sodium-potassium pump is an information processing element in brain computation". Frontiers in Physiology. 5 (472): 472. doi:10.3389/fphys.2014.00472. PMC 4274886. PMID 25566080.
- ^ Cannon C (July 2004). "Paying the Price at the Pump: Dystonia from Mutations in a Na+/K+-ATPase". Neuron. 43 (2): 153–154. doi:10.1016/j.neuron.2004.07.002. PMID 15260948.
- ^ Calderon DP, Fremont R, Kraenzlin F, Khodakhah K (March 2011). "The neural substrates of rapid-onset Dystonia-Parkinsonism". Nature Neuroscience. 14 (3): 357–65. doi:10.1038/nn.2753. PMC 3430603. PMID 21297628.
- ^ Forrest MD (2014). "Intracellular Calcium Dynamics Permit a Purkinje Neuron Model to Perform Toggle and Gain Computations Upon its Inputs". Frontiers in Computational Neuroscience. 8: 86. doi:10.3389/fncom.2014.00086. PMC 4138505. PMID 25191262.
- ^ Forrest MD (April 2015). "Simulation of alcohol action upon a detailed Purkinje neuron model and a simpler surrogate model that runs >400 times faster". BMC Neuroscience. 16 (27): 27. doi:10.1186/s12868-015-0162-6. PMC 4417229. PMID 25928094.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Forrest, Michael (April 2015). "the_neuroscience_reason_we_fall_over_when_drunk". Science 2.0.
- ^ a b Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, et al. (2016). "Consensus Paper: Neuroimmune Mechanisms of Cerebellar Ataxias". Cerebellum (Review). 15 (2): 213–32. doi:10.1007/s12311-015-0664-x. PMC 4591117. PMID 25823827.
- ^ Jaber M (2017). "The cerebellum as a major player in motor disturbances related to Autistic Syndrome Disorders". Encephale (Review). 43 (2): 170–175. doi:10.1016/j.encep.2016.03.018. PMID 27616580.
- ^ Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, Ullrich R, Villalta D, Volta U, Catassi C, Fasano A (2012). "Spectrum of gluten-related disorders: consensus on new nomenclature and classification". BMC Medicine (Review). 10: 13. doi:10.1186/1741-7015-10-13. PMC 3292448. PMID 22313950.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Hadjivassiliou M, Sanders DD, Aeschlimann DP (2015). "Gluten-related disorders: gluten ataxia". Dig Dis (Review). 33 (2): 264–8. doi:10.1159/000369509. PMID 25925933. S2CID 207673823.
- ^ Stucki DM, Ruegsegger C, Steiner S, Radecke J, Murphy MP, Zuber B, Saxena S (August 2016). "Mitochondrial impairments contribute to Spinocerebellar ataxia type 1 progression and can be ameliorated by the mitochondria-targeted antioxidant MitoQ" (PDF). Free Radic. Biol. Med. 97: 427–440. doi:10.1016/j.freeradbiomed.2016.07.005. PMID 27394174.
- ^ For references, see the extensive references and bibliography at the article on Cerebellar abiotrophy, linked at the beginning of this paragraph.
- ^ Mavroudis, IA; Fotiou, DF; Adipepe, LF; Manani, MG; Njau, SD; Psaroulis, D; Costa, VG; Baloyannis, SJ (November 2010). "Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer's disease". American Journal of Alzheimer's Disease & Other Dementias. 25 (7): 585–91. doi:10.1177/1533317510382892. PMID 20870670. S2CID 30688657.
- ^ Fekadu, Makonnen (27 March 2009). "Rabies encephalitis, Negri bodies within the cytoplasm of cerebellar Purkinje cell neurons". CDC/Frontal Cortex Inc. Retrieved 21 June 2013. Note: not peer-reviewed.
External links
- Cell Image Library - Purkinje
- Disorders of cerebellum
- NIF Search - Purkinje Cell via the Neuroscience Information Framework
Further reading
- Llinás R, Hess R (July 1976). "Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells". Proc. Natl. Acad. Sci. U.S.A. 73 (7): 2520–3. Bibcode:1976PNAS...73.2520L. doi:10.1073/pnas.73.7.2520. PMC 430632. PMID 1065905.
- Llinás R, Sugimori M (August 1980). "Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices". J. Physiol. 305: 171–95. doi:10.1113/jphysiol.1980.sp013357. PMC 1282966. PMID 7441552.
- Llinás RR, Sugimori M, Cherksey B (1989). "Voltage-dependent calcium conductances in mammalian neurons. The P channel". Ann. N. Y. Acad. Sci. 560 (1 Calcium Chann): 103–11. doi:10.1111/j.1749-6632.1989.tb24084.x. PMID 2545128. S2CID 84107834.
- Forrest, Michael (October 2014). Biophysics and computations of the cerebellar Purkinje neuron. CreateSpace. ISBN 978-1502454546.
