Coenzyme-B sulfoethylthiotransferase
| coenzyme-B sulfoethylthiotransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.8.4.1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, coenzyme-B sulfoethylthiotransferase, also known as methyl-coenzyme M reductase (MCR) or most systematically as 2-(methylthio)ethanesulfonate:N-(7-thioheptanoyl)-3-O-phosphothreonine S-(2-sulfoethyl)thiotransferase is an enzyme that catalyzes the final step in the formation of methane.[1] It does so by combining the hydrogen donor coenzyme B and the methyl donor coenzyme M. Via this enzyme, most of the natural gas on earth was produced. Ruminants (e.g. cows) produce methane because their rumens contain methanogenic prokaryotes (Archaea)[2][3] that encode and express the set of genes of this enzymatic complex.
The enzyme has two active sites, each occupied by the nickel-containing F430 cofactor.[4]
- + ⇌ CoM-S-S-CoB + methane
 |
 |
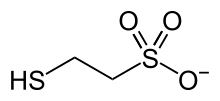
The two substrates of this enzyme are 2-(methylthio)ethanesulfonate and N-(7-mercaptoheptanoyl)threonine 3-O-phosphate; its two products are CoM-S-S-CoB and methane. 3-Nitrooxypropanol inhibits the enzyme.[5]
In some species, the enzyme reacts in reverse (a process called reverse methanogenesis), catalysing the anaerobic oxidation of methane, therefore removing it from the environment.[6] Such organisms are methanotrophs.
This enzyme belongs to the family of transferases, specifically those transferring alkylthio groups.
This enzyme participates in folate biosynthesis.[citation needed]
Structure
Coenzyme-B sulfoethylthiotransferase is a multiprotein complex made up of a pair of identical halves. Each half is made up of three subunits: α, β and γ,[7] also called McrA, McrB and McrG, respectively.
References
- ^ Stephen W., Ragdale (2014). "Chapter 6. Biochemistry of Methyl-Coenzyme M Reductase: The Nickel Metalloenzyme that Catalyzes the Final Step in Synthesis and the First Step in Anaerobic Oxidation of the Greenhouse Gas Methane". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 125–145. doi:10.1007/978-94-017-9269-1_6. PMID 25416393.
- ^ http://microbewiki.kenyon.edu/index.php/Bovine_Rumen#Methanogens
- ^ Whitford MF, Teather RM, Forster RJ (2001). "Phylogenetic analysis of methanogens from the bovine rumen". BMC Microbiology. 1: 5. doi:10.1186/1471-2180-1-5. PMC 32158. PMID 11384509.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Thauer RK (September 1998). "Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture". Microbiology. 144 (9): 2377–406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- ^ Hristov AN, Oh J, Giallongo F, Frederick TW, Harper MT, Weeks HL, Branco AF, Moate PJ, Deighton MH, Williams SR, Kindermann M, Duval S (August 2015). "An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production". Proceedings of the National Academy of Sciences of the United States of America. 112 (34): 10663–8. Bibcode:2015PNAS..11210663H. doi:10.1073/pnas.1504124112. PMC 4553761. PMID 26229078.
- ^ Hallam SJ, Putnam N, Preston CM, Detter JC, Rokhsar D, Richardson PM, DeLong EF (September 2004). "Reverse methanogenesis: testing the hypothesis with environmental genomics". Science. 305 (5689): 1457–62. Bibcode:2004Sci...305.1457H. doi:10.1126/science.1100025. PMID 15353801.
- ^ Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK (November 1997). "Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation". Science. 278 (5342): 1457–62. Bibcode:1997Sci...278.1457E. doi:10.1126/science.278.5342.1457. PMID 9367957.
Further reading
- Bobik TA, Olson KD, Noll KM, Wolfe RS (December 1987). "Evidence that the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate is a product of the methylreductase reaction in Methanobacterium". Biochemical and Biophysical Research Communications. 149 (2): 455–60. doi:10.1016/0006-291X(87)90389-5. PMID 3122735.
- Ellermann J, Rospert S, Thauer RK, Bokranz M, Klein A, Voges M, Berkessel A (September 1989). "Methyl-coenzyme-M reductase from Methanobacterium thermoautotrophicum (strain Marburg). Purity, activity and novel inhibitors". European Journal of Biochemistry. 184 (1): 63–8. doi:10.1111/j.1432-1033.1989.tb14990.x. PMID 2506016.
- Signor L, Knuppe C, Hug R, Schweizer B, Pfaltz A, Jaun B (October 2000). "Methane formation by reaction of a methyl thioether with a photo-excited nickel thiolate--a process mimicking methanogenesis in archaea". Chemistry. 6 (19): 3508–16. doi:10.1002/1521-3765(20001002)6:19<3508::AID-CHEM3508>3.3.CO;2-N. PMID 11072815.
