Coenzyme A

| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.472 |
| KEGG | |
| MeSH | Coenzyme+A |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H36N7O16P3S | |
| Molar mass | 767.535 |
| UV-vis (λmax) | 259.5 nm[1] |
| Absorbance | ε259 = 16.8 mM−1 cm−1 [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
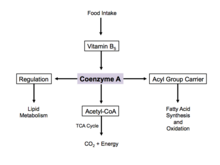
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP).[2]
In its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the anabolic and catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of pyruvate synthesis and degradation.[3]
Discovery of structure

Coenzyme A was identified by Fritz Lipmann in 1946,[4] who also later gave it its name. Its structure was determined during the early 1950s at the Lister Institute, London, together by Lipmann and other workers at Harvard Medical School and Massachusetts General Hospital.[5] Lipmann initially intended to study acetyl transfer in animals, and from these experiments he noticed a unique factor that was not present in enzyme extracts but was evident in all organs of the animals. He was able to isolate and purify the factor from pig liver and discovered that its function was related to a coenzyme that was active in choline acetylation.[6] The coenzyme was named coenzyme A to stand for "activation of acetate". In 1953, Fritz Lipmann won the Nobel Prize in Physiology or Medicine "for his discovery of co-enzyme A and its importance for intermediary metabolism".[6][7]
Biosynthesis
Coenzyme A is naturally synthesized from pantothenate (vitamin B5), which is found in food such as meat, vegetables, cereal grains, legumes, eggs, and milk.[8] In humans and most living organisms, pantothenate is an essential vitamin that has a variety of functions.[9] In some plants and bacteria, including Escherichia coli, pantothenate can be synthesised de novo and is therefore not considered essential. These bacteria synthesize pantothenate from the amino acid aspartate and a metabolite in valine biosynthesis.[10]
In all living organisms, coenzyme A is synthesized in a five-step process that requires four molecules of ATP, pantothenate and cysteine[11] (see figure):

- Pantothenate (vitamin B5) is phosphorylated to 4′-phosphopantothenate by the enzyme pantothenate kinase (PanK; CoaA; CoaX). This is the committed step in CoA biosynthesis and requires ATP.[10]
- A cysteine is added to 4′-phosphopantothenate by the enzyme phosphopantothenoylcysteine synthetase (PPCS; CoaB) to form 4'-phospho-N-pantothenoylcysteine (PPC). This step is coupled with ATP hydrolysis.[10]
- PPC is decarboxylated to 4′-phosphopantetheine by phosphopantothenoylcysteine decarboxylase (PPC-DC; CoaC)
- 4′-Phosphopantetheine is adenylated (or more properly, AMPylated) to form dephospho-CoA by the enzyme phosphopantetheine adenylyl transferase (PPAT; CoaD)
- Finally, dephospho-CoA is phosphorylated to coenzyme A by the enzyme dephosphocoenzyme A kinase (DPCK; CoaE). This final step requires ATP.[10]
Enzyme nomenclature abbreviations in parentheses represent eukaryotic and prokaryotic enzymes respectively. This pathway is regulated by product inhibition. CoA is a competitive inhibitor for Pantothenate Kinase, which normally binds ATP.[10] Coenzyme A, three ADP, one monophosphate, and one diphosphate are harvested from biosynthesis.[11]
New research shows that coenzyme A can be synthesized through alternate routes when intracellular coenzyme A level are reduced and the de novo pathway is impaired.[12] In these pathways, coenzyme A needs to be provided from an external source, such as food, in order to produce 4′-phosphopantetheine. Ectonucleotide pyrophosphates (ENPP) degrade coenzyme A to 4′-phosphopantetheine, a stable molecule in organisms. Acyl carrier proteins (ACP) (such as ACP synthase and ACP degradation) are also used to produce 4′-phosphopantetheine. This pathways allows for 4′-phosphopantetheine to be replenished in the cell and allows for the conversion to coenzyme A through enzymes, PPAT and PPCK.[13]
Commercial production
Coenzyme A is produced commercially via extraction from yeast, however this is an inefficient process (yields approximately 25 mg/kg) resulting in an expensive product. Various ways of producing CoA synthetically, or semi-synthetically have been investigated although none are currently operating at an industrial scale.[14]
Function
Fatty acid synthesis
Since coenzyme A is, in chemical terms, a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. It assists in transferring fatty acids from the cytoplasm to mitochondria. A molecule of coenzyme A carrying an acyl group is also referred to as acyl-CoA. When it is not attached to an acyl group, it is usually referred to as 'CoASH' or 'HSCoA'. This process facilitates the production of fatty acids in cells, which are essential in cell membrane structure.
Coenzyme A is also the source of the phosphopantetheine group that is added as a prosthetic group to proteins such as acyl carrier protein and formyltetrahydrofolate dehydrogenase.[15][16]
Energy production
Coenzyme A is one of five crucial coenzymes that are necessary in the reaction mechanism of the citric acid cycle. Its acetyl-coenzyme A form is the primary input in the citric acid cycle and is obtained from glycolysis, amino acid metabolism, and fatty acid beta oxidation. This process is the body's primary catabolic pathway and is essential in breaking down the building blocks of the cell such as carbohydrates, amino acids, and lipids.[17]
Regulation
When there is excess glucose, coenzyme A is used in the cytosol for synthesis of fatty acids.[18] This process is implemented by regulation of acetyl-CoA carboxylase, which catalyzes the committed step in fatty acid synthesis. Insulin stimulates acetyl-CoA carboxylase, while epinephrine and glucagon inhibit its activity.[19]
During cell starvation, coenzyme A is synthesized and transports fatty acids in the cytosol to the mitochondria. Here, acetyl-CoA is generated for oxidation and energy production.[18] In the citric acid cycle, coenzyme A works as an allosteric regulator in the stimulation of the enzyme pyruvate dehydrogenase.
New research has found that protein CoAlation plays an important role in regulation of the oxidative stress response. Protein CoAlation plays a similar role to S-glutathionylation in the cell, and prevents the irreversible oxidation of the thiol group in cysteine on the surface of cellular proteins, while also directly regulating enzymatic activity in response to oxidative or metabolic stress.[20]
Use in biological research
Coenzyme A is available from various chemical suppliers as the free acid and lithium or sodium salts. The free acid of coenzyme A is detectably unstable, with around 5% degradation observed after 6 months when stored at −20 °C,[21] and near complete degradation after 1 month at 37 °C.[22] The lithium and sodium salts of CoA are more stable, with negligible degradation noted over several months at various temperatures.[23] Aqueous solutions of coenzyme A are unstable above pH 8, with 31% of activity lost after 24 hours at 25 °C and pH 8. CoA stock solutions are relatively stable when frozen at pH 2–6. The major route of CoA activity loss is likely the air oxidation of CoA to CoA disulfides. CoA mixed disulfides, such as CoA-S–S-glutathione, are commonly noted contaminants in commercial preparations of CoA.[21] Free CoA can be regenerated from CoA disulfide and mixed CoA disulfides with reducing agents such as dithiothreitol or 2-mercaptoethanol.
Non-exhaustive list of coenzyme A-activated acyl groups
- Acetyl-CoA
- fatty acyl-CoA (activated form of all fatty acids; only the CoA esters are substrates for important reactions such as mono-, di-, and triacylglycerol synthesis, carnitine palmitoyl transferase, and cholesterol esterification)
- Acetoacetyl-CoA
- Coumaroyl-CoA (used in flavonoid and stilbenoid biosynthesis)
- Benzoyl-CoA
- Phenylacetyl-CoA
- Acyl derived from dicarboxylic acids
- Malonyl-CoA (important in chain elongation in fatty acid biosynthesis and polyketide biosynthesis)
- Succinyl-CoA (used in heme biosynthesis)
- Hydroxymethylglutaryl-CoA (used in isoprenoid biosynthesis)
- Pimelyl-CoA (used in biotin biosynthesis)
References
- ^ a b Dawson, Rex M. C.; Elliott, Daphne C.; Elliott, William H.; Jones, Kenneth M. (2002). Data for Biochemical Research (3rd ed.). Clarendon Press. ISBN 978-0-19-855299-4.
- ^ Daugherty, Matthew; Polanuyer, Boris; Farrell, Michael; Scholle, Michael; Lykidis, Athanasios; de Crécy-Lagard, Valérie; Osterman, Andrei (2002). "Complete Reconstitution of the Human Coenzyme: A Biosynthetic Pathway via Comparative Genomics". Journal of Biological Chemistry. 277 (24): 21431–21439. doi:10.1074/jbc.M201708200. PMID 11923312.
- ^ "Coenzyme A: when small is mighty". www.asbmb.org. Archived from the original on 2018-12-20. Retrieved 2018-12-19.
- ^ Lipmann, Fritz; Nathan O., Kaplan (1946). "A common factor in the enzymatic acetylation of sulfanilamide and of choline". Journal of Biological Chemistry. 162 (3): 743–744.
- ^ Baddiley, J.; Thain, E. M.; Novelli, G. D.; Lipmann, F. (1953). "Structure of Coenzyme A". Nature. 171 (4341): 76. Bibcode:1953Natur.171...76B. doi:10.1038/171076a0. PMID 13025483.
- ^ a b Kresge, Nicole; Simoni, Robert D.; Hill, Robert L. (2005-05-27). "Fritz Lipmann and the Discovery of Coenzyme A". Journal of Biological Chemistry. 280 (21): e18. ISSN 0021-9258.
- ^ "Fritz Lipmann – Facts". Nobelprize.org. Nobel Media AB. 2014. Retrieved 8 November 2017.
- ^ "Vitamin B5 (Pantothenic acid)". University of Maryland Medical Center. Retrieved 2017-11-08.
- ^ "Pantothenic Acid (Vitamin B5): MedlinePlus Supplements". medlineplus.gov. Retrieved 2017-12-10.
- ^ a b c d e Leonardi, Roberta; Jackowski, Suzanne (April 2007). "Biosynthesis of Pantothenic Acid and Coenzyme A". EcoSal Plus. 2 (2). doi:10.1128/ecosalplus.3.6.3.4. ISSN 2324-6200. PMC 4950986. PMID 26443589.
- ^ a b Leonardi, R.; Zhang, Y.-M.; Rock, C. O.; Jackowski, S. (2005). "Coenzyme A: back in action". Progress in Lipid Research. 44 (2–3): 125–153. doi:10.1016/j.plipres.2005.04.001. PMID 15893380.
- ^ de Villiers, Marianne; Strauss, Erick (October 2015). "Metabolism: Jump-starting CoA biosynthesis". Nature Chemical Biology. 11 (10): 757–758. doi:10.1038/nchembio.1912. ISSN 1552-4469. PMID 26379022.
- ^ Sibon, Ody C. M.; Strauss, Erick (October 2016). "Coenzyme A: to make it or uptake it?". Nature Reviews Molecular Cell Biology. 17 (10): 605–606. doi:10.1038/nrm.2016.110. ISSN 1471-0080. PMID 27552973.
- ^ Mouterde, Louis M. M.; Stewart, Jon D. (19 December 2018). "Isolation and Synthesis of One of the Most Central Cofactors in Metabolism: Coenzyme A". Organic Process Research & Development. 23: 19–30. doi:10.1021/acs.oprd.8b00348.
- ^ Elovson, J.; Vagelos, P. R. (July 1968). "Acyl carrier protein. X. Acyl carrier protein synthetase". J. Biol. Chem. 243 (13): 3603–11. PMID 4872726.
- ^ Strickland, K. C.; Hoeferlin, L. A.; Oleinik, N. V.; Krupenko, N. I.; Krupenko, S. A. (January 2010). "Acyl carrier protein-specific 4′-phosphopantetheinyl transferase activates 10-formyltetrahydrofolate dehydrogenase". Journal of Biological Chemistry. 285 (3): 1627–1633. doi:10.1074/jbc.M109.080556. PMC 2804320. PMID 19933275.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). "Molecular Biology of the Cell (4th ed.): Chapter 2: How Cells Obtain Energy from Food".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Shi, Lei; Tu, Benjamin P. (April 2015). "Acetyl-CoA and the Regulation of Metabolism: Mechanisms and Consequences". Current Opinion in Cell Biology. 33: 125–131. doi:10.1016/j.ceb.2015.02.003. ISSN 0955-0674. PMC 4380630. PMID 25703630.
- ^ Berg, Jeremy M.; Tymoczko, John L.; Stryer, Lubert (2002). "Acetyl Coenzyme A Carboxylase Plays a Key Role in Controlling Fatty Acid Metabolism".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Tsuchiya, Yugo; Peak-Chew, Sew Yeu; Newell, Clare; Miller-Aidoo, Sheritta; Mangal, Sriyash; Zhyvoloup, Alexander; Baković, Jovana; Malanchuk, Oksana; Pereira, Gonçalo C. (2017-07-15). "Protein CoAlation: a redox-regulated protein modification by coenzyme A in mammalian cells". Biochemical Journal. 474 (14): 2489–2508. doi:10.1042/BCJ20170129. ISSN 0264-6021. PMC 5509381. PMID 28341808.
- ^ a b Dawson, R. M. C. (1989). Data for biochemical research. Oxford: Clarendon Press. pp. 118–119. ISBN 978-0-19-855299-4.
- ^ "Datasheet for free acid coenzyme A" (PDF). Oriental Yeast Co., LTD.
- ^ "Datasheet for lithium salt coenzyme A" (PDF). Oriental Yeast Co., LTD.
Bibliography
- Nelson, David L.; Cox, Michael M. (2005). Lehninger: Principles of Biochemistry (4th ed.). New York: W .H. Freeman. ISBN 978-0-7167-4339-2.
