Barium peroxide

| |
| |
| Names | |
|---|---|
| IUPAC name
barium peroxide
| |
| Other names
Barium binoxide,
Barium dioxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.754 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaO2 | |
| Molar mass | 169.33 g/mol (anhydrous) 313.45 (octahydrate) |
| Appearance | Grey-white crystalline (anhydrous) colorless solid (octahydrate) |
| Odor | odorless |
| Density | 5.68 g/cm3 (anhydrous) 2.292 g/cm3 (octahydrate) |
| Melting point | 450 °C (842 °F; 723 K) |
| Boiling point | 800 °C (1,470 °F; 1,070 K) (decomposes to BaO & O2.[1]) |
| anhydrous 0.091 g/100 mL (20 °C) octahydrate 0.168 g/cm3 | |
| Solubility | dissolves with decomposition in acid |
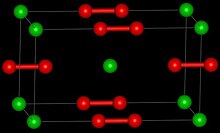
| Structure | |
| Tetragonal [2] | |
| D174h, I4/mmm, tI6 | |
| 6 | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium peroxide is the inorganic compound with the formula BaO2. This white solid (gray when impure) is one of the most common inorganic peroxides, and it was the first peroxide compound discovered. Being an oxidizer and giving a vivid green colour upon ignition (as do all barium compounds), it finds some use in fireworks; historically, it was also used as a precursor for hydrogen peroxide.[3]
Structure
Barium peroxide is a peroxide, containing O2−
2 subunits. The solid is isomorphous to calcium carbide, CaC2.
Preparation and use
Barium peroxide arises by the reversible reaction of O2 with barium oxide. The peroxide forms around 500 °C and oxygen is released above 820 °C.[1]
- 2 BaO + O2 ⇌ 2 BaO2
This reaction is the basis for the now-obsolete Brin process for separating oxygen from the atmosphere. Other oxides, e.g. Na2O and SrO, behave similarly.[4]
In another obsolete application, barium peroxide was once used to produce hydrogen peroxide via its reaction with sulfuric acid:[3]
- BaO2 + H2SO4 → H2O2 + BaSO4
The insoluble barium sulfate is filtered from the mixture.
Footnotes
- ^ a b Accommodation of Excess Oxygen in Group II Monoxides - S.C. Middleburgh, R.W. Grimes and K.P.D. Lagerlof Journal of the American Ceramic Society 2013, Volume 96, pages 308–311. doi:10.1111/j.1551-2916.2012.05452.x
- ^ Massalimov, I. A.; Kireeva, M. S.; Sangalov, Yu. A. (2002). "Structure and Properties of Mechanically Activated Barium Peroxide". Inorganic Materials. 38 (4): 363–366. doi:10.1023/A:1015105922260.
- ^ a b Harald Jakob; Stefan Leininger; Thomas Lehmann; Sylvia Jacobi; Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.

