Batrachochytrium dendrobatidis
| Batrachochytrium dendrobatidis | |
|---|---|

| |
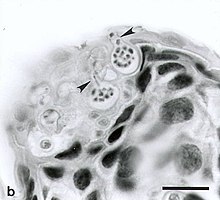
| Zoosporangia of Batrachochytrium dendrobatidis growing on a freshwater arthropod (a) and on algae (b). The scale bars represent 30 µm. | |
| Scientific classification | |
| Missing taxonomy template (fix): | Batrachochytrium dendrobatidis |
| Binomial name | |
| Batrachochytrium dendrobatidis Longcore, Pessier & D.K. Nichols (1999)
| |
Batrachochytrium dendrobatidis, (bə-TRAY-koh-KIT-ri-um DEN-droh-bə-teye-dis) also known as Bd or the amphibian chytrid fungus, is a chytrid fungus that causes the disease chytridiomycosis in amphibians. In the decade after it was first discovered in 1998,[1] the disease devastated amphibian populations around the world, in a global decline towards multiple extinctions, part of the Holocene extinction. A recently described second species, B. salamandrivorans, also cause chytridiomycosis and death in salamanders.
Some amphibian species appear to have an innate capacity to withstand chytridiomycosis infection. Even within species that generally succumb, some populations survive, possibly demonstrating that these traits or alleles of species are being subjected to evolutionary selection.
Etymology
The generic name is derived from the Greek words batrachos (frog) and chytra (earthen pot), while the specific epithet is derived from the genus of frogs from which the original confirmation of pathogenicity was made (Dendrobates).[2]
Systematics
Batrachochytrium dendrobatidis was until recently considered the single species of the genus Batrachochytrium. The initial classification of the pathogen as a chytrid was based on zoospore ultrastructure. DNA analysis of the ssu-rDNA has corroborated the view, with the closest match to Chytridium confervae. A second species of Batrachochytrium was discovered in 2013: B. salamandrivorans, which mainly affects salamanders and also causes chytridiomycosis.[3] B. salamandrivorans differs from B. dendrobatidis primarily in the formation of germ tubes in vitro, the formation of colonial thalli with multiple sporangia in vivo, and a lower thermal preference.[3]
Morphology

B. dendrobatidis infects the keratinized skin of amphibians. The fungus in the epidermis has a thallus bearing a network of rhizoids and smooth-walled, roughly spherical, inoperculate (without an operculum) sporangia. Each sporangium produces a single tube to discharge spores.
Zoospore structure
Zoospores of B. dendrobatidis, which are typically 3-5 µm in size, have an elongate–ovoidal body with a single, posterior flagellum (19-20 µm long), and possess a core area of ribosomes often with membrane-bound spheres of ribosomes within the main ribosomal mass.[2] A small spur has been observed, located at the posterior of the cell body, adjacent to the flagellum, but this may be an artifact in the formalin-fixed specimens. The core area of ribosomes is surrounded by a single cisterna of endoplasmic reticulum, two to three mitochondria, and an extensive microbody–lipid globule complex. The microbodies closely appose and almost surround four to six lipid globules (three anterior and one to three laterally), some of which appear bound by a cisterna. Some zoospores appear to contain more lipid globules (this may have been a result of a plane-of-sectioning effect, because the globules were often lobed in the zoospores examined). A rumposome has not been observed.[2]
Flagellum structure
A nonfunctioning centriole lies adjacent to the kinetosome. Nine interconnected props attach the kinetosome to the plasmalemma, and a terminal plate is present in the transitional zone. An inner ring-like structure attached to the tubules of the flagellar doublets within the transitional zone has been observed in transverse section. No roots associated with the kinetosome have been observed. In many zoospores, the nucleus lies partially within the aggregation of ribosomes and was invariably situated laterally. Small vacuoles and a Golgi body with stacked cisternae occurred within the cytoplasm outside the ribosomal area. Mitochondria, which often contain a small number of ribosomes, are densely staining with discoidal cristae.[2]
Life cycle
B. dendrobatidis has two primary life stages: a sessile, reproductive zoosporangium and a motile, uniflagellated zoospore released from the zoosporangium. The zoospores are known to be active only for a short period of time, and can travel short distances of one to two centimeters.[4] However, the zoospores are capable of chemotaxis, and can move towards a variety of molecules that are present on the amphibian surface, such as sugars, proteins and amino acids.[5] B. dendrobatidis also contains a variety of proteolytic enzymes and esterases that help it digest amphibian cells and use amphibian skin as a nutrient source.[6] Once the zoospore reaches its host, it forms a cyst underneath the surface of the skin, and initiates the reproductive portion of its life cycle. The encysted zoospores develop into zoosporangia, which may produce more zoospores that can reinfect the host, or be released into the surrounding aquatic environment.[7] The amphibians infected with these zoospores are shown to die from cardiac arrest.[citation needed]
Physiology
B. dendrobatidis can grow within a wide temperature range (4-25 °C), with optimal temperatures being between 17-25 °C.[8] The wide temperature range for growth, including the ability to survive at 4 °C gives the fungus the ability to overwinter in its hosts, even where temperatures in the aquatic environments are low. The species does not grow well above temperatures of 25 °C, and growth is halted above 28 °C.[8] Infected red-eyed treefrogs (Litoria chloris) recovered from their infections when incubated at a temperature of 37 °C.[9]
Varying forms
B. dendrobatidis has occasionally been found in forms distinct from its traditional zoospore and sporangia stages. For example, before the 2003 European heat wave that decimated populations of the water frog Rana lessonae through chytridiomycosis, the fungus existed on the amphibians as spherical, unicellular organisms, confined to minute patches (80–120 meters across). These organisms, unknown at the time, were subsequently identified as B. dendrobatidis. Characteristics of the organisms were suggestive of encysted zoospores; they may have embodied a resting spore, a saprobe, or a parasitic form of the fungus that is non-pathogenic.[10]
Habitat and relationship to amphibians
The fungus grows on amphibian skin and produces aquatic zoospores.[11] It is widespread and ranges from lowland forests to cold mountain tops. It is sometimes a non-lethal parasite and possibly a saprophyte. The fungus is associated with host mortality in highlands or during winter, and becomes more pathogenic at lower temperatures.[12]
Geographic distribution
It has been suggested that B. dendrobatidis originated in Africa and subsequently spread to other parts of the world by trade in African clawed frogs (Xenopus laevis).[13] In this study, 697 archived specimens of three species of Xenopus, previously collected from 1879 to 1999 in southern Africa were examined. The earliest case of chytridiomycosis was found in a X. laevis specimen from 1938. The study also suggests that chytridiomycosis had been a stable infection in southern Africa from 23 years prior to finding any infected outside of Africa.[13]
American bullfrogs (Lithobates catesbeiana), also widely distributed, are also thought to be carriers of the disease due to their inherent low susceptibility to B. dendrobatidis infection.[14][15] The bullfrog often escapes captivity and can establish feral populations where it may introduce the disease to new areas.[4] It has also been shown that B. dendrobatidis can survive and grow in moist soil and on bird feathers, suggesting that B. dendrobatidis may also be spread in the environment by birds and transportation of soils.[16] Infections have been linked to mass mortalities of amphibians in North America, South America, Central America, Europe and Australia.[17][18][19] B. dendrobatidis has been implicated in the extinction of the sharp-snouted day frog (Taudactylus acutirostris) in Australia.[20]
A wide variety of amphibian hosts have been identified as being susceptible to infection by B. dendrobatidis, including wood frogs (Lithobates sylvatica),[21] the mountain yellow-legged frog (Lithobates muscosa)[22] the southern two-lined salamander (Eurycea cirrigera),[23] San Marcos Salamander (Eurycea nana) Texas Salamander (Eurycea neotenes) Blanco River Springs Salamander (Eurycea pterophila) Barton Springs Salamander (Eurycea sosorum) Jollyville Plateau Salamander (Eurycea tonkawae) [24] Ambystoma jeffersonianum,[25] the western chorus frog (Pseudacris triseriata), the southern cricket frog (Acris gryllus), the eastern spadefoot toad (Scaphiopus holbrooki), the southern leopard frog (Lithobates sphenocephala),[26] the Rio Grande Leopard frog (Lithobates berlandieri),[27] and the Sardinian newt (Euproctus platycephalus).[28]
Southeast Asia
While most studies concerning B. dendrobatidis have been performed in various locations across the world, the presence of the fungus in Southeast Asia remains a relatively recent development. The exact process through which the fungus was introduced to Asia is not known, however, as mentioned above, it has been suggested transportation of asymptomatic carrier species (e.g. Lithobates catesbeianus, the American Bullfrog) may be a key component in the dissemination of the fungus, at least in China.[29] Initial studies demonstrated the presence of the fungus on island states/countries such as Hong Kong,[30] Indonesia,[31] Taiwan,[26] and Japan.[32] Soon thereafter, mainland Asian countries such as Thailand,[33] South Korea,[34] and China[35] reported incidences of B. dendrobatidis among their amphibian populations. Much effort has been put into classifying herpetofauna in countries like Cambodia, Vietnam, and Laos where new species of frogs, toads, and other amphibians and reptiles are being discovered on a frequent basis. Scientists simultaneously are swabbing herptofauna in order to determine if these newly discovered animals possess traces of the fungus.
In Cambodia, a study showed B. dendrobatidis to be prevalent throughout the country in areas near Phnom Penh (in a village <5 km), Sihanoukville (frogs collected from the local market), Kratie (frogs collected from streets around the town), and Siem Reap (frogs collected from a national preserve: Angkor Centre for Conservation of Biodiversity).[36] Another study in Cambodia questioned the potential anthropological impact in the dissemination of B. dendrobatidis on local amphibian populations in 3 different areas in relation to human interaction: low (an isolated forest atop a mountain people rarely visit), medium (a forest road ~15 km from a village that is used at least once a week), and high (a small village where humans interact with their environment on a daily basis). Using quantitative PCR, evidence of B. dendrobatidis was found in all 3 sites with the highest percentage of amphibians positive for the fungus from the forest road (medium impact; 50%), followed by the mountain forest (low impact; 44%) and village (high impact; 36%).[37] Human influence most likely explains detection of the fungus in the medium and high areas, however it does not provide an adequate explanation why even isolated amphibians were positive for B. dendrobatidis. This may go unanswered until more research is performed on transmission of the fungus across landscapes. Another study in French Guiana reports widespread infection, with 8 of 11 sites sampled being positive for B. dendrobatidis infection for at least one species.[38] This study suggests that Bd is more widespread than previously thought.
See also
References
- ^ Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. (July 1998). "Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America". Proceedings of the National Academy of Sciences of the United States of America. 95 (15): 9031–6. doi:10.1073/pnas.95.15.9031. PMC 21197. PMID 9671799.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Longcore JE, Pessier AP, Nichols DK (1129). "Batrachochytrium Dendrobatidis gen. et sp. nov, a chytrid pathogenic to amphibians". Mycologia. 91 (2): 219–227. doi:10.2307/3761366. JSTOR 3761366.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M. C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; Pasmans, F. (2013). "Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians". Proceedings of the National Academy of Sciences of the United States of America. 110 (38): 15325–15329. doi:10.1073/pnas.1307356110. PMC 3780879. PMID 24003137.
- ^ a b Garner TW, Perkins MW, Govindarajulu P, Seglie D, Walker S, Cunningham AA, Fisher MC (September 2006). "The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Lithobates catesbeiana". Biol. Lett. 2 (3): 455–9. doi:10.1098/rsbl.2006.0494. PMC 1686185. PMID 17148429.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moss AS, Reddy NS, Dortaj IM, San Francisco MJ (2008). "Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants". Mycologia. 100 (1): 1–5. doi:10.3852/mycologia.100.1.1. PMID 18488347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Symonds EP, Trott DJ, Bird PS, Mills P (2008). "Growth characteristics and enzyme activity in Batrachochytrium dendrobatidis isolates". Mycopathologia. 166 (3): 143–147. doi:10.1007/s11046-008-9135-y. PMID 18568420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Berger L, Hyatt AD, Speare R, Longcore JE (December 2005). "Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis". Dis. Aquat. Org. 68 (1): 51–63. doi:10.3354/dao068051. PMID 16465834.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Piotrowski JS, Annis S, Longcore JE (2004). "Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians". Mycologia. 96 (1): 9–15. doi:10.2307/3761981. JSTOR 3761981.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Woodhams DC, Alford RA, Marantelli G (June 2003). "Emerging disease of amphibians cured by elevated body temperature". Dis. Aquat. Org. 55 (1): 65–7. doi:10.3354/dao055065. PMID 12887256.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Di Rosa, Ines et al. "The Proximate Cause of Frog Declines?" Nature 447.31 (2007) E4-E5.
- ^ Ron, S. R. Predicting the distribution of the amphibian pathogen B. dendrobatidis in the New World. Biotropica 37 209-221 (2005)
- ^ Daszak, P.; Cuningham, A. A. & Hyatt, A.D. Infection disease and amphibian population declines. Divers. Distrib. 9 141-150 (2003).
- ^ a b Weldon C, du Preez LH, Hyatt AD, Muller R, Spears R (December 2004). "Origin of the amphibian chytrid fungus". Emerging Infect. Dis. 10 (12): 2100–5. doi:10.3201/eid1012.030804. PMID 15663845.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kats LB, Ferrer RP (2003). "Alien predators and amphibian declines: review of two decades of science and the transition to conservation". Diversity and Distributions. 9: 99–110. doi:10.1046/j.1472-4642.2003.00013.x.
- ^ Daszak P, Strieby A, Cunningham AA, Longcore JE, Brown CC, Porter D (2004). "Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians". Herpetological Journal. 14: 201–207.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson ML, Speare R (July 2005). "Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment". Dis. Aquat. Org. 65 (3): 181–6. doi:10.3354/dao065181. PMID 16119886.
- ^ Lips KR (1999). "Mass mortality and population declines of anurans at an upland site in western Panama". Conservation Biology. 13 (1): 117–125. doi:10.1046/j.19993-2449.2022.97185.x.
- ^ Daszak P, Cunningham AA, Hyatt AD (2003). "Infectious disease and amphibian population declines" (PDF). Diversity and Distributions. 9: 141–50. doi:10.1046/j.1472-4642.2003.00016.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Herrera RA, Steciow MM, Natale GS (2005). "Chytrid fungus parasitizing the wild amphibian Leptodactylus ocellatus (Anura: Leptodactylidae) in Argentina". Diseases of Aquatic Organisms. 64 (3): 247–52. doi:10.3354/dao064247. PMID 15997823.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P (2006). "The decline of the sharp-snouted day frog (Taudactylus acutiostris): the first documented case of extinction by infection in a free-ranging wildlife species?". EcoHealth. 3: 35–40. doi:10.1007/s10393-005-0012-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reeves MK (2008). "Batrachochytrium dendrobatidis in wood frogs (Lithobates sylvatica) from Three National Wildlife Refuges in Alaska, USA". Herpetological Review. 39 (1): 68–70.
- ^ Andre SE, Parker J, Briggs CJ (2008). "Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Lithobates muscosa)". Journal of Wildlife Diseases. 44 (3): 716–720. doi:10.7589/0090-3558-44.3.716. PMID 18689660.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Byrne MW, Davie EP, Gibbons JW (2008). "Batrachochytrium dendrobatidis occurrence in Eurycea cirrigera". Southeastern Naturlaist. 7 (3): 551–555. doi:10.1656/1528-7092-7.3.551.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gaertner JP, Forstner MRJ, O'Donnell L, Hahn D (2009). "Detection of Batrachochytrium dendrobatidis in endemic salamander species from Central Texas". EcoHealth. 6 (1): 20–26. doi:10.1007/s10393-009-0229-x. PMID 19424755.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brodman R, Briggler JT (2008). "Batrachochytrium dendrobatidis in Ambystoma jeffersonianum larvae in southern Indiana". Herpetological Review. 39 (3): 320–321.
- ^ a b Lehtinen RM, Kam Y-C Richards CL (2008). "Preliminary surveys for Batrachochytrium dendrobatidis in Taiwan". Herpetological Review. 39 (3): 317–318.
- ^ Lovich R, Ryan MJ, Pessier AP, CLaypool B (2008). "Infection with the fungus Batrachochytrium dendrobatidis in a non-native Lithobates berlandieri below sea level in the Coachella Valley, California, USA". Herpetological Review. 39 (3): 315–317.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bovero S, Sotgiu G, Angelini C, Doglio S, Gazzaniga E, Cunningham AA, Garner TWJ (2008). "Detection of chytridiomycosis caused by Batrachochytrium dendrobatidis in the endangered sardinian newt (Euproctus platycephalus) in Southern Sardinia, Italy". Journal of Wildlife Diseases. 44 (3): 712–715. doi:10.7589/0090-3558-44.3.712. PMID 18689659.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bai, C., T. W. Garner, and Y. Li (2010). "First evidence of Batrachochytrium dendrobatidis in China: discovery of chytridiomycosis in introduced American bullfrogs and native amphibians in the Yunnan Province, China". Dis Aqua Org. 92: 241–244.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rowley J, Chan SK, Tang WS, Speare R, Skerratt LF, Alford RA, Cheung KS, Ho CY, Campbell R (2007). "Survey for the amphibianchytrid Batrachochytrium dendrobatidis in Hong Kong in native amphibians and in the international amphibian trade". Diseases of Aquatic Organisms. 78: 87–95. doi:10.3354/dao01861.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kusrini, MD, Skerratt LF, Garland S, Berger L, Endarwin W (2008). "Chytridiomycosis in frogs of Mount Gede Pangrango, Indonesia". Diseases of Aquatic Organisms. 82: 187–194. doi:10.3354/dao01981. PMID 19244970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fisher MC, Garner TW, Walker SF (2009). "Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis inspace, time, and host". Annual Review of Microbiology. 63: 291–310. doi:10.1146/annurev.micro.091208.073435. PMID 19575560.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McLeod, DS, Sheridan JA, Jiraungkoorskul W, Khonsue W (2008). "A survey for chytrid fungus in Thai amphibians". Raffles Bulletin of Zoology. 56: 199–204.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yang, H., H. Baek, R. Speare, R. Webb, S. Park, T. Kim, K.C. Lasat er, S. Shin, S. Son, J. Park, M. Min, Y. Kim, K. Na, H. Lee, and S. Park (2008). "First detection of the amphibian chytrid fungus Batrachochytrium dendrobatidis in free-ranging populations of amphibians on mainland Asia: survey in South Korea". Dis Aqua Org. 86: 9–13. doi:10.3354/dao02098.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wei, Y., K. Xu, D.-Z. Zhu, X.-F. Chen, and X.-L. Wang (2010). "First Early-spring survey for Batrachochytrium dendrobatidis in wild Rana dybowskii in Heilongjiang Province, China". Dis Aqua Org. 92: 241–244. doi:10.3354/dao02172.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gaertner JP, Mendoza JA, Forstner MRJ, Neang T, Hahn D (2011). "Detection of Batrachochytrium dendrobatidis in frogs from different locations in Cambodia". Herpetological Review. 42: 546–548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mendoza JA, Gaertner JP, Holden J, Forstner MRJ, Hahn D (2011). "Detection of Batrachochytrium dendrobatidis on amphibians in Pursat Province, Cambodia". Herpetological Review. 42: 542–545.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Courtois EA, Gaucher P, Chave J, Schmeller DS (2015). "Widespread Occurrence of Bd in French Guiana, South America". PLOS ONE.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Daszak, Peter; Berger L; Cunningham AA; Hyatt AD; Green DE; Speare R. (1999). "Emerging Infectious Diseases and Amphibian Population Declines". Emerging Infectious Diseases. 5 (6): 735–748. doi:10.3201/eid0506.990601. PMC 2640803. PMID 10603206.
- Johnson, Megan L.; Speare, Richard (August 2003). "Survival of Batrachochytrium dendrobatidis in Water: Quarantine and Disease Control Implications". Emerging Infectious Diseases. 9 (8): 915–921. doi:10.3201/eid0908.030145.
