Benzyl benzoate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ascabin, Ascabiol, Ascarbin, Tenutex, others |
| Other names | phenylmethyl ester, benzoic ester |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.003 |
| Chemical and physical data | |
| Formula | C14H12O2 |
| Molar mass | 212.248 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.118 g/cm3 g/cm3 |
| Melting point | 18 °C (64 °F) |
| Boiling point | 323 °C (613 °F) |
| Solubility in water | insoluble mg/mL (20 °C) |
| |
| |
Benzyl benzoate is an organic compound which is used as a medication and insect repellent.[1] As a medication it is used to treat scabies and lice.[2] For scabies either permethrin or malathion is typically preferred.[3] It is applied to the skin as a lotion.[2] Typically two to three applications are needed.[2] It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.[4]
Side effects may include irritation of the skin.[2] It is not recommended in children.[3] It is also used in other animals; however, it is considered toxic to cats.[1] How it works is unclear.[5]
Benzyl benzoate was first studied medically in 1918.[1] It is on the World Health Organization's List of Essential Medicines.[6] Benzyl benzoate is sold under the brand name Scabanca among others and is available as a generic medication.[1][3] It is not available for medical use in the United States.[1]
Uses
[edit]Medical
[edit]Benzyl benzoate is an effective and inexpensive topical treatment for human scabies.[7] It has vasodilating and spasmolytic effects and is present in many asthma and whooping cough drugs.[8] It is also used as an excipient in some testosterone-replacement medications (like Nebido) for treating hypogonadism.[9]
Benzyl benzoate is used as a topical acaricide, scabicide, and pediculicide in veterinary hospitals.[10]
Non-medical
[edit]Benzyl benzoate is used as a repellent for chiggers, ticks, and mosquitoes.[10] It is also used as a dye carrier, solvent for cellulose derivatives, plasticizer, and fixative in the perfume industry.[11]
Side effects
[edit]Benzyl benzoate has low acute toxicity in laboratory animals. It is rapidly hydrolyzed to benzoic acid and benzyl alcohol. Benzyl alcohol is subsequently metabolized to benzoic acid. The conjugates of benzoic acid (hippuric acid and the glucuronide of benzoic acid) are rapidly eliminated in urine.[1] When given in large doses to laboratory animals, benzyl benzoate can cause hyperexcitation, loss of coordination, ataxia, convulsions, and respiratory paralysis.[10]
Benzyl benzoate can be a skin irritant when used as a topical scabicide.[7] Overdose can result in blistering and hives or a rash can occur as an allergic reaction.[12][13]
As an excipient in some testosterone-replacement injectable medications, benzyl benzoate has been reported as a cause of anaphylaxis in a case in Australia.[14] Bayer includes this report in information for health professionals and recommends that physicians "should be aware of the potential for serious allergic reactions" to preparations of this type.[9] In Australia, reports to ADRAC, which evaluates reports of adverse drug reactions for the Therapeutic Goods Administration, show several reports of allergic issues since the anaphylaxis case from 2011.
Chemistry
[edit]It is an organic compound with the formula C6H5CH2O2CC6H5. It is the ester of benzyl alcohol and benzoic acid. It forms either a viscous liquid or solid flakes and has a weak, sweet-balsamic odor. It occurs in a number of blossoms (e. g. tuberose, hyacinth) and is a component of Balsam of Peru and Tolu balsam.[11][4]
Production
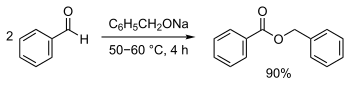
[edit]Benzyl benzoate is produced industrially by the reaction of sodium benzoate with benzyl chloride in the presence of a base, or by transesterification of methyl benzoate and benzyl alcohol.[8] It is a byproduct of benzoic acid synthesis by toluene oxidation.[11] It can also be synthesized by the Tishchenko reaction, using benzaldehyde with sodium benzyloxide (generated from sodium and benzyl alcohol) as catalyst:[15][16]
Chittajit Mohan Dhar was a pioneer in the production of dimethyl phthalate and benzyl benzoate in India.[17]
References
[edit]- ^ a b c d e f Knowles CO (1991). "22.4.2 Benzyl Benzoate". In Hayes WJ, Laws ER (eds.). Handbook of Pesticide Toxicology, Volume 3: Classes of Pesticides. Academic Press. pp. 1505–1508. ISBN 0123341639.
- ^ a b c d World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 311. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c British National Formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 836. ISBN 9780857111562.
- ^ a b Fahlbusch K, Hammerschmidt F, Panten J, Pickenhagen W, Schatkowski D, Bauer K, et al. (15 January 2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a11_141. ISBN 3527306730.
- ^ Bowman DD (2009). Georgis' Parasitology for Veterinarians. Elsevier Health Sciences. p. 262. ISBN 978-1416044123.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b Burns DA (2010), "Diseases Caused by Arthropods and Other Noxious Animals", in Breathnach SM, Griffiths CE, Cox N, Burns T (eds.), Rook's Textbook of Dermatology, vol. 2 (8th ed.), Wiley-Blackwell, p. 38.41
- ^ a b Brühne F, Wright E (15 June 2000). "Benzyl Alcohol". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a04_001. ISBN 3527306730.
- ^ a b "Nebido Monograph – Information for Health Care Professionls". Bayer. 2016. Archived from the original on 19 October 2016. Retrieved 19 October 2016.
- ^ a b c Shaikh J (2005). "Benzyl Benzoate". In Wexler P (ed.). Encyclopedia of Toxicology. Vol. 1 (2nd ed.). Elsevier. pp. 264–265.
- ^ a b c Maki T, Takeda K (15 June 2000). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a03_555. ISBN 3527306730.
- ^ "Benzyl Benzoate (Topical route)". PubMed Health at the United States National Library of Medicine. 1 October 2016. Archived from the original on 10 September 2017. Retrieved 19 October 2016.
- ^ "Benzocaine-Benzyl Benzoate Topical: Side Effects". WebMD. 2016. Archived from the original on 19 October 2016. Retrieved 19 October 2016.
- ^ Ong GS, Somerville CP, Jones TW, Walsh JP (2012). "Anaphylaxis triggered by benzyl benzoate in a preparation of depot testosterone undecanoate". Case Reports in Medicine. 2012: 384054. doi:10.1155/2012/384054. PMC 3261473. PMID 22272209. 384054.
- ^ Brühne F, Wright E (15 October 2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3527306732.
- ^ Kamm O, Kamm WF (1922). "Benzyl benzoate". Organic Syntheses. 2: 5. doi:10.15227/orgsyn.002.0005; Collected Volumes, vol. 1, p. 104.
- ^ "Bombay Times Newspaper- Chittajit Mohan Dhar and Professor Humayun Kabir"