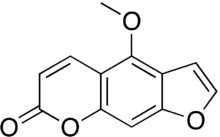
Bergapten
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.913 |
| Chemical and physical data | |
| Formula | C12H8O4 |
| Molar mass | 216.19 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bergapten (5-methoxypsoralen) is a psoralen (also known as furocoumarins) found in bergamot essential oil, in other citrus essential oils,[1] and in grapefruit juice.[2] It is the chemical in bergamot oil that causes phototoxicity.[3] Bergapten-free bergamot essential oil or synthetics are now used in perfumery.
A known use of bergapten is in the synthesis of Fraxinol.[4]
References
- ^ Calvarano I.; Calvarano M.; Gionfriddo F.; Bovalo F.; Postorino E. (1995). "HPLC profile of citrus essential oils from different species and geographic origin". Essenze Derivati agrumari. 65: 488–502.
- ^ Sakamaki N.; Nakazato M.; Matsumoto H.; Hagino K.; Hirata K.; Ushiyama H. (2008). "Contents of furanocoumarins in grapefruit juice and health foods". Journal of the Food Hygienic Society of Japan. 49 (4): 326–331. doi:10.3358/shokueishi.49.326. PMID 18787320.
- ^ Francesco Gionfriddo; Enrico Postorino; Giuseppe Calabrò (2004). "Elimination of Furocoumarins in Bergamot Peel Oil". Perfumer & Flavorist. 29.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Schönberg, Alexander; Badran, Nasry; Starkowsky, Nicolas A. (1955). "Furo-chromones and -Coumarins. XII. Synthesis of Fraxinol from Bergapten and of Baicalein from Visnagin". Journal of the American Chemical Society. 77 (20): 5390–5392. doi:10.1021/ja01625a055. ISSN 0002-7863.
