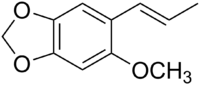
Carpacin
Appearance
| |
| |
| Names | |
|---|---|
| IUPAC name
(E)-2-Methoxy-4,5-methylenedioxypropenylbenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12O3 | |
| Molar mass | 192.21 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Carpacin is a naturally occurring organic compound first isolated from the Carpano tree (Cinnamomum sp.), from which it derives its name. It is a biosynthetic precursor of the more complex lignan-dimer, carpanone.[1] Carpacin is classified as a phenylpropanoid.
Carpacin has been prepared synthetically from sesamol[2] and has been studied for potential use as an insecticide,[3] an antidepressant, and an inhibitor of carcinogenesis.[2]
References
- ^ J. Mohandas, M. Slaytor, T.R. Watson (1969). "Trans-2-methoxy-4,5-methylenedioxypropenylbenzene (carpacin) from a Cinnamomum sp. from Bougainville". Aust. J. Chem. 22 (8): 1803–1804. doi:10.1071/CH9691803.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tsui-Hwa Tseng, Yen-Min Tsheng, Yean-Jang Lee, Hsing-Ling Hsu (2000). "Total Synthesis of Carpacin and Its Geometric Isomer as a Cancer Chemopreventer" (PDF). Journal of the Chinese Chemical Society. 47: 1165–1169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ BH Alexander, SI Gertler, RT Brown, TA Oda, M Beroza (1959). "Synthesis of Methylenedioxyphenyl Compounds from Isosafrole and Sesamol". J. Org. Chem. 24 (10): 1504. doi:10.1021/jo01092a029.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
