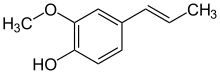
Isoeugenol
| |
| Names | |
|---|---|
| IUPAC name
2-Methoxy-4-[(E)-prop-1-enyl]phenol
| |
| Other names
4-Hydroxy-3-methoxy-1-propenylbenzene
2-Methoxy-4-propenylphenol 4-Propenylguaiacol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.356 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Appearance | Oily liquid |
| Density | 1.080 |
| Melting point | −10 °C (14 °F; 263 K) |
| Boiling point | 266 °C (511 °F; 539 K) |
| 810 mg/L [2] | |
| Solubility | Soluble in most organic solvents[3] |
| Vapor pressure | 0.0135 mmHg[4] |
| Pharmacology | |
| QN01AX94 (WHO) | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isoeugenol is a propenyl-substituted guaiacol. A phenylpropanoid, it occurs in the essential oils of plants such as ylang-ylang (Cananga odorata), and is a component of wood smoke and liquid smoke. It can be synthesized from eugenol and has been used in the manufacture of vanillin. It may occur as either the cis (Z) or trans (E) isomer. Trans (E) isoeugenol is crystalline while cis (Z) isoeugenol is a liquid.[6] Isoeugenol is one of several phenolic compounds responsible for the mold-inhibiting effect of smoke on meats and cheeses.[7]
Allergy[edit]
Some individuals experience a hives-like reaction to long-term exposure to Isoeugenol, which is named as Fragrance in the ingredients of consumer products such as soaps, shampoos and detergents, bath tissue, and topical cosmetic applications. Sensitivity to isoeugenol (Fragrance) may be identified with a clinical patch test.[8]
References[edit]
- ^ The Merck Index, 12th edition, Merck & Co, Whitehouse Station, New Jersey, USA, 1996.
- ^ HERA; HERA Risk Assessment of Isoeugenol (Draft). Human and Environmental Risk Assessment on ingredients of Household Cleaning Products. Isoeugenol (CAS 97-54-1). Available from, as of June 23, 2012: http://www.heraproject.com/
- ^ Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 15th Edition. John Wiley & Sons, Inc. New York, NY 2007., p. 710
- ^ NIST; NIST Chemistry WebBook. Phenol, 2-methoxy-4-(1-propenyl)- (97-54-1). NIST Standard Reference Database No. 69, June 2005 Release. Washington, DC: US Sec Commerce. Available from, as of June 25, 2012: http://webbook.nist.gov
- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-10-26]". Health Canada. 26 October 2023. Retrieved 3 January 2024.
- ^ Merck Index
- ^ WENDORFF, WILLIAM L.; RIHA, WILLIAM E.; MUEHLENKAMP, EMILY (November 1993). "Growth of Molds on Cheese Treated with Heat or Liquid Smoke". Journal of Food Protection. 56 (11): 963–966. doi:10.4315/0362-028X-56.11.963. PMID 31113088.
- ^ AllergEAZE. "PF137 Isoeugenol" (PDF). AllergEAZE. Retrieved 2015-08-25.
