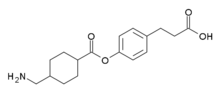
Cetraxate
Appearance
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H23NO4 |
| Molar mass | 305.36 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
Cetraxate (INN) is an oral gastrointestinal medication which has a cytoprotective effect.[1] Double-blind clinical trials in patients with gastric ulcer compared the effects of cetraxate with those of the drug gefarnate, a common standard drug treatment for gastric ulcer. Endoscopic examinations of patients treated with cetraxate showed cure rates of 28% after 4 weeks, 61% after 8 weeks, and 73% after 12 weeks of treatment, significantly higher than cure rates seen with gefarnate at both 8 and 12 weeks.[2]
References
- ^ Kurebayashi Y, Ikeda T, Osada Y (January 1988). "Cytoprotective action of cetraxate against HCl.ethanol-induced gastric lesion in rats". Jpn. J. Pharmacol. 46 (1): 17–25. doi:10.1254/jjp.46.17. PMID 3367546.
- ^ Ishimori A, Yamagata S, Taima T (1979). "Effect of p-hydroxyphenyl-propionic ester of tranexamic acid hydrochloride (Cetraxate) on peptic ulcer. Multi-center clinical study". Arzneimittelforschung. 29 (10): 1625–32. PMID 391240.
