Chloroplatinic acid
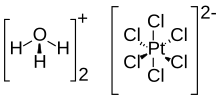
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Dihydrogen hexachloroplatinate(2–)
| |
| Other names
Hexachloroplatinic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.267 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H2PtCl6 | |
| Molar mass | 409.81 g/mol |
| Appearance | Reddish brown solid |
| Density | 2.431 g/cm3 |
| Melting point | 60 °C (140 °F; 333 K) |
| Boiling point | decomposes |
| highly soluble | |
| Structure | |
| Anti-fluorite. | |
| octahedral | |
| 0 D | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Hexachloropalladic acid |
Other cations
|
Potassium hexachloroplatinate, Ammonium hexachloroplatinate, Rubidium hexachloroplatinate, Caesium hexachloroplatinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloroplatinic acid or hexachloroplatinic acid is an inorganic compound with the formula [H3O]2[PtCl6](H2O)x. A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2−
6).[1][2][3] The compound is also available as the hexahydrate.
Production

Chloroplatinic acid is produced by dissolving platinum metal sponge in aqua regia. Brownish red crystals can be isolated by evaporating this solution to a syrup.[4]
- Pt + 4 HNO3 + 6 HCl → "H2PtCl6" + 4 NO2 + 4 H2O
A related procedure gives the hexahydrate, H2PtCl6(H2O)6.[5]
Alternative methods have been heavily investigated, but the older literature can be unreliable.[6]
Reactions
When heated, hexachloroplatinic acid decomposes first to platinum(IV) chloride, and for this reason heating of hexachloroplatinic acid can result in insoluble platinum compounds.[1]
- (H3O)2PtCl6·nH2O → PtCl4 + 2 HCl + (n + 2) H2O
Applications
Potassium determination
Chloroplatinic acid was popularized for the determination of potassium. The potassium is selectively precipitated as potassium chloroplatinate. Determinations were done in 85% (v/v) alcohol solutions with excess platinate ions, and the precipitated product was weighed. Potassium could be detected for solutions as dilute as 0.02 to 0.2% (m/v).[7]
This method for determination of potassium was advantageous vs. the sodium cobaltinitrite method used previously, since it required a single precipitation reaction.[8] Today, the concentration of potassium is determined by a number of methods such as ion-selective electrodes, flame photometry, ICP-AES or ICP-MS.
Purification of platinum
Treatment with an ammonium salt, such as ammonium chloride, precipitates solid ammonium hexachloroplatinate,.[4] Heating the ammonium salt in hydrogen reduces it to elemental platinum. Platinum is often isolated from ores or recycled from residues thus.[9]
Catalysis
Like many platinum compounds, chloroplatinic acid is used in catalysis. This compound was first reported by John Speier and colleagues from Dow Corning Corporation to catalyze the addition of silyl hydrides to olefins, hydrosilylation. Early test reactions used isopropanol solutions of trichlorosilane (SiHCl3) with pentenes. Prior work on the addition of silanes to alkenes required radical reactions that were inefficient.[10][11] As well as with Karstedt's catalyst, Speier's catalyst enjoys widespread use for hydrosilylation, the main drawback is the deliquescent properties of the catalyst.[12]
It is generally agreed that chloroplatinic acid is a precursor to the actual catalyst. A possible role for colloidal platinum or zero-valent complexes has also been considered.[13]
Related compounds
Chloroplatinic acid prepared from aqua regia is occasionally contaminated with nitrosonium hexachloroplatinate, (NO)2PtCl6. This species is obtained by the reaction of nitrosyl chloride (NOCl) and platinum metal.[14]
References
- ^ a b Schweizer, A. E.; Kerr, G. T. (1978). "Thermal Decomposition of Hexachloroplatinic Acid". Inorg. Chem. 17 (8): 2326–2327. doi:10.1021/ic50186a067.
- ^ Holleman; Wiberg (2001). Inorganic Chemistry (First ed.). New York: Academic Press. ISBN 0-12-352651-5.
- ^ The related palladium compound, [H3O]2[PdCl6], is extremely unstable and has not been isolated in pure form.Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (Second ed.). New York: Elsevier Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- ^ a b Kauffman, George B. (1967). "Ammonium Hexachloroplatinate(IV)". Inorganic Syntheses. 9: 182–185. doi:10.1002/9780470132401.ch51.
- ^ Grube, H. (1963). "Hexachloroplatinic(IV) Acid". In Brauer, G. (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 2 (2nd ed.). New York: Academic Press. p. 1569.
- ^ Rudnick, Paul; Cooke, R. D. (1917). "The Preparation of Hydrochloroplatinic Acid by means of Hydrogen Peroxide". J. Am. Chem. Soc. 39 (4): 633–635. doi:10.1021/ja02249a011.
- ^ Smith, G. F.; Gring, J. L. (1933). "The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium". J. Am. Chem. Soc. 55 (10): 3957–3961. doi:10.1021/ja01337a007.
- ^ G. F. Smith; J. L. Gring (1933). "The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium". J. Am. Chem. Soc. 55 (10): 3957–3961. doi:10.1021/ja01337a007.
- ^ Cotton, S. A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 0-7514-0413-6.
- ^ Speier, J. L.; Webster, J. A.; Barnes, G. H. (1957). "The Addition of Silicon Hydrides to Olefinic Double Bonds. Part II. The Use of Group VIII Metal Catalysts". J. Am. Chem. Soc. 79 (4): 974–979. doi:10.1021/ja01561a054.
- ^ Saam, John C.; Speier, John L. (1958). "The Addition of Silicon Hydrides to Olefinic Double Bonds. Part III. The Addition to Non-terminal Olefins in the Presence of Chloroplatinic Acid". J. Am. Chem. Soc. 80 (15): 4104–4106. doi:10.1021/ja01548a073.
- ^ Mukund P. Sibi,"Hydrogen Hexachloroplatinate(IV)" Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. doi:10.1002/047084289X.rh038
- ^ Lewis, L. N.; Sy, K. G.; Bryant, G. L.; Donahue, P. E. (1991). "Platinum-catalyzed hydrosilylation of alkynes". Organometallics. 10 (10): 3750–3759. doi:10.1021/om00056a055.
- ^ Moravek, R. T.; Kauffman, G. B.; Mahmood, T. (1967). "Nitrosyl Hexachloroplatinate(IV)". Inorganic Syntheses. 9: 217–220. doi:10.1002/9780470132555.ch63.

