Ideain
| |
| Names | |
|---|---|
| IUPAC name
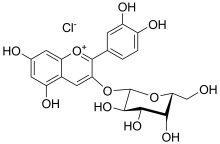
(2S,4S,5R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol;chloride
| |
| Other names
Cyanidin 3-O-galactoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C21H21ClO11 (chloride), C21H21O11+ (cation) | |
| Molar mass | 484.83 g/mol (chloride), 449.38 g/mol (cation) |
| UV-vis (λmax) | 528 nm (in methanol) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.
Natural occurrences
[edit]Ideain is the main anthocyanin in red-skinned[1] or red-fleshed (for example Weirouge)[2] apple varieties. It is also found in Chinese hawthorn fruits (Crataegus spp.).[3] It is also the pigment in the copper beech (cultivar of Fagus sylvatica), that was identified in 1932.[4]
While it is only one in the many anthocyanins present in bilberries (Vaccinium myrtillus)[5] and cranberries (Vaccinium macrocarpon),[6] it is the main anthocyanin in lingonberries (Vaccinium vitis-idaea).[7]
Quintinia serrata, the tawheowheo, a species of evergreen trees endemic to New Zealand, has different patterns of anthocyanins (cyanidin 3-O-glucoside and cyanidin 3-O-galactoside) in its leaves to protect the shade-adapted chloroplasts from direct sun light.[8]
References
[edit]- ^ Determination of Polyphenolic Profiles of Basque Cider Apple Varieties Using Accelerated Solvent Extraction. R. M. Alonso-Salces, E. Korta, A. Barranco, L. A. Berrueta, B. Gallo and F. Vicente, J. Agric. Food Chem., 2001, volume 49, pages 3761−3767, doi:10.1021/jf010021
- ^ Chemical quality parameters and anthocyanin pattern of red-fleshed Weirouge apples. E. Sadilova, F. C. Stintzing and R. Carle, Journal of Applied Botany and Food Quality, 2006, volume 80, pages 82-87 (link to abstract Archived 2016-09-21 at the Wayback Machine)
- ^ Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography–electrospray ionisation mass spectrometry. Pengzhan Liu, Heikki Kallio, Deguo Lü, Chuansheng Zhou, Baoru Yang, Food Chemistry, Volume 127, Issue 3, 1 August 2011, Pages 1370–1377, doi:10.1016/j.foodchem.2011.01.103
- ^ Robinson, R.; Smith, H. (1955). "Anthocyanins of the Leaf of the Copper Beech (Fagus sylvatica) and the Fruit of the Cultivated Strawberry (Fragaria virginiana)". Nature. 175: 634. doi:10.1038/175634a0.
- ^ Qualitative and quantitative evaluation of vaccinium myrtillus anthocyanins by high-resolution gas chromatography and high-performance liquid chromatography. A. Baj, E. Bombardelli, B. Gabetta and E.M. Martinelli, Journal of Chromatography A, Volume 279, 25 November 1983, Pages 365-372, doi:10.1016/S0021-9673(01)93636-2
- ^ Urinary Excretion of Anthocyanins in Humans after Cranberry Juice Ingestion. Ryoko Ohnishi, Hideyuki Ito, Naoki Kasajima, Miyuki Kaneda, Reiko Kariyama, Hiromi Kumon, Tsutomu Hatano and Takashi Yoshida, Bioscience, Biotechnology, and Biochemistry, 2006, Volume 70, Issue 7, pages 1681-1687, {{DOI:10.1271/bbb.60023}}
- ^ Urinary Excretion of the Main Anthocyanin in Lingonberry (Vaccinium vitis-idaea), Cyanidin 3-O-Galactoside, and Its Metabolites. Henna-Maria Lehtonen, Milla Rantala, Jukka-Pekka Suomela, Matti Viitanen and Heikki Kallio, J. Agric. Food Chem., 2009, 57 (10), pages 4447–4451, doi:10.1021/jf900894k
- ^ Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. Kevin S. Gould, Kenneth R. Markham, Richard H. Smith and Jessica J. Goris, J. Exp. Bot., 2000, volume 51, issue 347, pages 1107-1115, doi:10.1093/jexbot/51.347.1107
