Hydroxymethylfurfural

| |

| |
| Names | |
|---|---|
| IUPAC name
5-(hydroxymethyl)-2-furaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.595 |
| KEGG | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.11 g/mol |
| Density | 1.29 g/cm3 |
| Melting point | 30 to 34 °C (86 to 93 °F; 303 to 307 K) |
| Boiling point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) (1 mbar) |
| UV-vis (λmax) | 282 nm[1] |
| Related compounds | |
Related furan-2-carbaldehydes
|
Furfural |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxymethylfurfural (HMF), also 5-(Hydroxymethyl)furfural, is an organic compound derived from dehydration of certain sugars.[2][3][4] This yellow low-melting solid is highly water-soluble. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups. HMF has been identified in a wide variety of baked goods. HMF, which is derived from hexoses, is a potential "carbon-neutral" feedstock for fuels and chemicals.[5]
Production and reactions
Related to the production of furfural, HMF is produced from sugars. It arises via the dehydration of fructose.[6][7] Treatment of fructose with acids followed by liquid-liquid extraction into organic solvents such as methyl isobutyl ketone. The conversion is affected by various additives such as DMSO, 2-butanol, and polyvinyl pyrrolidone, which minimize the formation of side product. Ionic liquids facilitate the conversion of fructose to HMF.[8] When hexoses are hydrolyzed with hydrochloric acid, 5-chloromethylfurfural is produced instead of HMF.
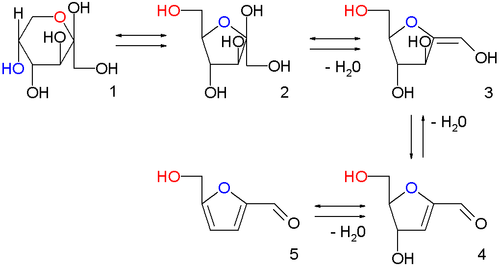
In the image above are displayed in a series of chemical equilibria: fructopyranose 1, fructofuranose 2, two intermediate stages of dehydration (not isolated) 3,4 and finally HMF 5.
Chromous chloride catalyzes the direct conversion of both fructose (yielding 90%+) and glucose (yielding 70%+) into an HMF.[9] Cellulose can also be converted into HMF (yielding 55% at 96% purity), in a process that proceeds via the intermediacy of glucose and fructose.[10][11]
HMF can be converted to 2,5-dimethylfuran (DMF), a liquid that is a potential biofuel with a greater energy content than bioethanol. Oxidation of HMF gives 2,5-furandicarboxylic acid, which has been proposed as a replacement for terephthalic acid in the production of polyesters. Reduction gives 2,5-bis(hydroxymethyl)furan. Acid-catalysed hydrolysis converts HMF into gamma-valerolactone, with loss of formic acid.[12]
Occurrence in food
HMF is practically absent in fresh food, but it is naturally generated in sugar-containing food during heat-treatments like drying or cooking. Along with many other flavor- and color-related substances, HMF is formed in the Maillard reaction as well as during caramelization. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF.[13] HMF is a well known component of baked goods. Upon toasting bread, the amount increases from 14.8 (5 min.) to 2024.8 mg/kg (60 min).[3]
It is a good wine storage time−temperature marker,[14] especially in sweet wines such as Madeira[15] and those sweetened with grape concentrate arrope.[16]
As an unwanted component

HMF can be found in low amounts in honey, fruit-juices and UHT-milk. Here, as well as in vinegars, jams, alcoholic products or biscuits HMF can be used as an indicator for excess heat-treatment. For instance, fresh honey contains less than 15 mg/kg—depending on pH-value and temperature and age,[17] and the codex alimentarius standard requires that honey have less than 40 mg/kg HMf to guarantee that the honey has not undergone heating during processing, except for tropical honeys which must be below 80 mg/kg.
Higher quantities of HMF are found naturally in coffee and dried fruit. Several types of roasted coffee contained between 300 – 2900 mg/kg HMF.[18] Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found,[19] bakery-products contained between 4.1 – 151 mg/kg HMF.[20]
It can be found in glucose syrup.
HMF can form in high-fructose corn syrup (HFCS), levels around 20 mg/kg HMF were found, increasing during storage or heating.[17] This is a problem for American beekeepers because they use HFCS as a source of sugar when there are not enough nectar sources to feed honeybees, and HMF is toxic to them. Adding bases such as soda ash or potash to neutralize the HFCS slows the formation of HMF.[17]
Depending on production-technology and storage, levels in food vary considerably. To evaluate the contribution of a food to HMF intake, its consumption-pattern has to be considered. Coffee is the food that has a very high relevance in terms of levels of HMF and quantities consumed.
HMF is a natural component in heated food but usually present in low concentrations. The daily intake of HMF may underlie high variations due to individual consumption-patterns. It has been estimated that in a western diet, in the order of magnitude of 5 – 10 mg of HMF are ingested per day from food.[13]
In former times, HMF was used in food for flavoring purposes, but in Europe this practice now is suspended. HMF is also found in cigarette smoke.[21]
Biomedical
A major metabolite in humans is 5-hydroxymethyl-2-furoic acid (HMFA), which is excreted in urine. HMF can also be metabolized to 5-sulfoxymethylfurfural (SMF), which is highly reactive and can form adducts with DNA or proteins. In vitro tests and studies on rats suggest potential toxicity and carcinogenicity of HMF.[citation needed] In humans, no correlation between intakes of HMF and disease is known.
HMF has been found to bind specifically with intracellular sickle hemoglobin (HbS). Preliminary in vivo studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood.[22] Under the development code Aes-103, HMF has been considered for the treatment of sickle cell disease.[23]
Quantification
Today, HPLC with UV-detection is the reference-method (e.g. DIN 10751-3). Classic methods for the quantification of HMF in food use photometry. The method according to White is a differential UV-photometry with and without sodium bisulphite-reduction of HMF (AOAC 980.23). Winkler photometric method is a colour-reaction using p-toluidine and barbituric acid (DIN 10751-1). Photometric test may be unspecific as they may detect also related substances, leading to higher results than HPLC-measurements. Test-kits for rapid analyses are also available (e.g. Refelctoquant HMF, Merck KGaA).[24][25]
History
This organic compound was first prepared from inulin using oxalic acid.[26] It was examined by French chemist Louis Maillard in 1912 in studies on non-enzymatic reactions of glucose. Its conversion to myriad organic compounds, e.g., solvents, polymer precursors, and biofuels has been regularly studied since the 1950s. In the 1980s, the role of acids in its formation was elucidated, especially means of avoiding the formation of humins.[3]
Other
HMF is an intermediate in the titration of hexoses in the Molisch's test. In the related Bial's test for pentoses, the hydroxymethylfurfural from hexoses may give a muddy-brown or gray solution, but this is easily distinguishable from the green color of pentoses.
AMF,[27] acetoxymethyl furfural, is also bio-derived green platform chemicals as an alternative to HMF.
References
- ^ The Determination of HMF in Honey with an Evolution Array UV-Visible Spectrophotometer. Nicole Kreuziger Keppy and Michael W. Allen, Ph.D., Application note 51864, Thermo Fisher Scientific, Madison, WI, USA (article)
- ^ Malgorzata E. Zakrzewska, Ewa Bogel-Lukasik, Rafal Bogel-Lukasik "Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfurals-A Promising Biomass-Derived Building Block" Chem. Rev., 2011, volume 111, 397. doi:10.1021/cr100171a
- ^ a b c Andreia A. Rosatella, Svilen P. Simeonov, Raquel F. M. Frade, Carlos A. M. Afonso "Critical Review 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological Properties, Synthesis and Synthetic Applications" Green Chem., 2011, volume 13, 754. doi:10.1039/c0gc00401d
- ^ Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources Robert-Jan van Putten, Jan C. van der Waal, Ed de Jong, Carolus B. Rasrendra, Hero J. Heeres, and Johannes G. de Vries Chemical Reviews 2013, vol. 113, pp. 1499–1597. doi:10.1021/cr300182k
- ^ Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chem. Rev. 106 (9): 4044–98. doi:10.1021/cr068360d. PMID 16967928.MIT Technology Review
- ^ Yuriy Román-Leshkov; Juben N. Chheda; James A. Dumesic (2006). "Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose". Science. 312 (5782): 1933–1937. doi:10.1126/science.1126337. PMID 16809536.
- ^ Synthesis of 5-(Hydroxymethyl)furfural (HMF) Svilen P. Simeonov, Jaime A. S. Coelho and Carlos A. M. Afonso Org. Synth. 2016, 93, 29 doi:10.15227/orgsyn.093.0029
- ^ Ståhlberg, T.; Fu, W.; Woodley, J. M.; Riisager, A. "Synthesis of 5-(Hydroxymethyl)furfural in Ionic Liquids: Paving the Way to Renewable Chemicals" ChemSusChem. 2011, Volume 4, pages 451–458. doi:10.1002/cssc.201000374
- ^ Haibo Zhao; Johnathan E. Holladay; Heather Brown; Z. Conrad Zhang (2007). "Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural". Science. 316 (5782): 1597–1600. doi:10.1126/science.1141199. PMID 17569858.
- ^ Su, Yu; Brown, Heather M.; Huang, Xiwen; Zhou, Xiao-Dong; Amonette, James E.; Zhang, Z. Conrad (2009). "Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical". Applied Catalysis A: General. 361: 117. doi:10.1016/j.apcata.2009.04.002.
- ^ A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, "5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications", Green Chemistry 2011, vol. 13, 754-793. doi:10.1039/c0gc00401d
- ^ van Putten, R.-J., van der Waal, J. C., de Jong, E., Rasrendra, C. B., Heeres, H. J.,de Vries, J. G., "Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources", Chem. Rev. 2013, 113, 1499.
- ^ a b Arribas-Lorenzo, G; Morales, FJ (2010). "Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population". Food and Chemical Toxicology. 48 (2): 644–9. doi:10.1016/j.fct.2009.11.046. PMID 20005914.
- ^ Kinetics of Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial Sparkling Wines. A. Serra-Cayuela, M. Jourdes, M. Riu-Aumatell, S. Buxaderas, P.-L. Teissedre and E. López-Tamames, J. Agric. Food Chem., 2014, volume 62, issue 5, pages 1159–1166, doi:10.1021/jf403281y
- ^ Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. V. Pereira, F.M. Albuquerque, A.C. Ferreira, J. Cacho and J.C. Marques, Food Research International, Volume 44, Issue 1, January 2011, Pages 71–76, doi:10.1016/j.foodres.2010.11.011
- ^ Hydroxymethylfurfural in California wines. Maynard A. Amerine, Journal of Food Science, May 1948, Volume 13, Issue 3, pages 264–269, doi:10.1111/j.1365-2621.1948.tb16621.x
- ^ a b c Ruiz-Matute, AI; Weiss, M; Sammataro, D; Finely, J; Sanz, ML (2010). "Carbohydrate composition of high-fructose corn syrups (HFCS) used for bee feeding: effect on honey composition". Journal of Agricultural and Food Chemistry. 58 (12): 7317–22. doi:10.1021/jf100758x. PMID 20491475.
- ^ Murkovic, M; Pichler, N (2006). "Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine". Molecular Nutrition & Food Research. 50 (9): 842–6. doi:10.1002/mnfr.200500262. PMID 16917810.
- ^ Husøy, T; Haugen, M; Murkovic, M; Jöbstl, D; Stølen, LH; Bjellaas, T; Rønningborg, C; Glatt, H; Alexander, J (2008). "Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure". Food and Chemical Toxicology. 46 (12): 3697–702. doi:10.1016/j.fct.2008.09.048. PMID 18929614.
- ^ Ramírez-Jiménez, A; Garcı́a-Villanova, Belén; Guerra-Hernández, Eduardo (2000). "Hydroxymethylfurfural and methylfurfural content of selected bakery products". Food Research International. 33 (10): 833. doi:10.1016/S0963-9969(00)00102-2.
- ^ Rufían-Henares, JA; De La Cueva, SP (2008). "Assessment of hydroxymethylfurfural intake in the Spanish diet". Food Additives & Contaminants: Part A. 25 (11): 1306–12. doi:10.1080/02652030802163406. PMID 19680837.
- ^ Abdulmalik, O; Safo, MK; Chen, Q; Yang, J; Brugnara, C; Ohene-Frempong, K; Abraham, DJ; Asakura, T (2005). "5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells". British journal of haematology. 128 (4): 552–61. doi:10.1111/j.1365-2141.2004.05332.x. PMID 15686467.
- ^ "Aes-103 Drug Development". AesRx.
- ^ Schultheiss, J.; Jensen, D.; Galensa, R. (2000). "Determination of aldehydes in food by high-performance liquid chromatography with biosensor coupling and micromembrane suppressors". Journal of Chromatography A. 880: 233. doi:10.1016/S0021-9673(99)01086-9.
- ^ Gaspar, Elvira M.S.M.; Lucena, Ana F.F. (2009). "Improved HPLC methodology for food control – furfurals and patulin as markers of quality". Food Chemistry. 114 (4): 1576. doi:10.1016/j.foodchem.2008.11.097.
- ^ G. Dull, Chemiker Zeitung, 1895, 216.
- ^ Kang, Eun-Sil; Hong, Yeon-Woo; Chae, Da Won; Kim, Bora; Kim, Baekjin; Kim, Yong Jin; Cho, Jin Ku; Kim, Young Gyu (13 April 2015). "From Lignocellulosic Biomass to Furans via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural". ChemSusChem. 8 (7): 1179–1188. doi:10.1002/cssc.201403252. ISSN 1864-564X.