Memory T cell
This article needs attention from an expert in Science. The specific problem is: difficult to follow, too technical. (November 2017) |

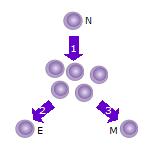
2. Some of the T cell clones will differentiate into effector T cells (E) that will perform the function of that cell (e.g. produce cytokines in the case of helper T cells or invoke cell killing in the case of cytotoxic T cells).
3. Some of the cells will form memory T cells (M) that will survive in an inactive state in the host for a long period of time until they re-encounter the same antigen and reactivate.
Memory T cells are a subset of infection- and cancer-fighting T cells (also known as a T lymphocyte) that have previously encountered and responded to their cognate antigen; thus, the term antigen-experienced T cell is often applied. Such T cells can recognize foreign invaders, such as bacteria or viruses, as well as cancer cells. Memory T cells have become "experienced" by having encountered antigen during a prior infection, encounter with cancer, or previous vaccination. At a second encounter with the invader, memory T cells can reproduce to mount a faster and stronger immune response than the first time the immune system responded to the pathogen that entered the body. This behaviour is utilized in T lymphocyte proliferation assays, which can reveal exposure to specific antigens.
Sub-populations
Historically, memory T cells were thought to belong to either the effector or central memory subtypes, each with their own distinguishing set of cell surface markers (see below).[1] Subsequently, numerous additional populations of memory T cells were discovered including tissue-resident memory T (Trm) cells, stem memory TSCM cells, and virtual memory T cells. The single unifying theme for all memory T cell subtypes is that they are long-lived and can quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen. By this mechanism they provide the immune system with "memory" against previously encountered pathogens. Memory T cells may be either CD4+ or CD8+ and usually express CD45RO.[2]
Memory T cell subtypes:
- Central memory T cells (TCM cells) express CD45RO, C-C chemokine receptor type 7 (CCR7), and L-selectin (CD62L). Central memory T cells also have intermediate to high expression of CD44. This memory subpopulation is commonly found in the lymph nodes and in the peripheral circulation. (Note- CD44 expression is usually used to distinguish murine naive from memory T cells).
- Effector memory T cells (TEM cells) express CD45RO but lack expression of CCR7 and L-selectin. They also have intermediate to high expression of CD44. These memory T cells lack lymph node-homing receptors and are thus found in the peripheral circulation and tissues.[3] TEMRA stands for terminally differentiated effector memory cells re-expressing CD45RA, which is a marker usually found on naive T cells.[4]
- Tissue resident memory T cells (TRM) occupy tissues (skin, lung, gastrointestinal tract, etc.) without recirculating. One cell surface marker that has been associated with TRM is the integrin αeβ7. These cells are thought to play a major role in protective immunity against pathogens.[5][6] Dysfunctional TRM cells have been implicated in autoimmune diseases, such as psoriasis, rheumatoid arthritis, inflammatory bowel disease.[6] Specific to TRMs are genes involved in lipid metabolism, being highly active, roughly 20- to 30-fold more active than in other types of T-cells.[6]
- Virtual memory T cells (TVM) differ from the other memory subsets in that they do not originate following a strong clonal expansion event. Thus, although this population as a whole is abundant within the peripheral circulation, individual virtual memory T cell clones reside at relatively low frequencies. One theory is that homeostatic proliferation gives rise to this T cell population. Although CD8 virtual memory T cells were the first to be described,[7] it is now known that CD4 virtual memory cells also exist.[8] Some have suggested that antigen-inexperienced memory T cells should be separated into ‘innate memory’ T cells and ‘virtual memory’ T cells.[9]
There have been numerous other subpopulations of memory T cells suggested. For example, in the mouse, Sendai virus specific CD8+ T-cells low on CD43 expression mounted a higher memory recall response suggesting that memory CD8 T-cells can also be distinguished from activated effector CD8 T-cells using CD43 marker.[10] Other investigators have studied Stem memory TSCM cells. Like naive T cells, TSCM cells are CD45RO−, CCR7+, CD45RA+, CD62L+ (L-selectin), CD27+, CD28+ and IL-7Rα+, but they also express large amounts of CD95, IL-2Rβ, CXCR3, and LFA-1, and show numerous functional attributes distinctive of memory cells.[11]
Function
Antigen-specific memory T cells against viruses or other microbial molecules can be found in both TCM and TEM subsets. Although most information is currently based on observations in the cytotoxic T cells (CD8-positive) subset, similar populations appear to exist for both the helper T cells (CD4-positive) and the cytotoxic T cells.
- central memory (TCM). The TCM cells are thought to contain some properties associated with memory cells stem cells. TCM display a capacity for self-renewal due to high levels of phosphorylation of an important transcription factor known as STAT5.[12] In mice, TCM cells have been shown to confer superior protection against viruses,[13] bacteria,[13] and cancer[14] in several different model systems compared with TEM cells.
- two highly related effector memory sub-types, which strongly express genes for molecules essential to the cytotoxic function of CD8 T cells:
- effector memory (TEM)
- effector memory RA (TEMRA)
- More recently, antigen-experienced CD8+ T cells with apparent self-renewal capabilities have been described in mice.[15][16] This population, now termed stem cell memory (TSCM), can be identified by the markers CD44(low)CD62L(high)CD122(high)sca-1(+) and are capable of generating TCM and TEM subsets while maintaining themselves. In preclinical studies, adoptively transferred TSCM confer superior immunity compared with other T memory subsets.[16] Whether such a population is found in humans is the subject of active investigation.
- After infection or inflammatory challenge, central memory CD8+ T cells rapidly traffic into nonlymphoid tissues, this cellular migration is driven by interleukin-15 stimulated enzymatic synthesis of core 2 O-glycans, which generates functional ligands for E- and P-selectins. In fact interleukin-15 stimulated expression of glycosyltransferase enzymes is largely a feature of TCM CD8+ T cells and this allows central memory T cell to selectively migrate out of the circulation and into nonlymphoid tissues. Therefore the entry of memory CD8+ T cells into inflamed, nonlymphoid tissues is primarily restricted to TCM cells that have the capacity to synthesize core 2 O-glycans.[17]
See also
References
- ^ Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (October 1999). "Two subsets of memory T lymphocytes with distinct homing potentials and effector functions". Nature. 401 (6754): 708–12. doi:10.1038/44385. PMID 10537110.
- ^ Akbar AN, Terry L, Timms A, Beverley PC, Janossy G (April 1988). "Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells". Journal of Immunology. 140 (7): 2171–8. PMID 2965180.
- ^ Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF (November 2005). "Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets". Journal of Immunology. 175 (9): 5895–903. doi:10.4049/jimmunol.175.9.5895. PMID 16237082.
- ^ Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G (July 2008). "Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people". Immunity & Ageing. 5 (6): 6. doi:10.1186/1742-4933-5-6. PMC 2515281. PMID 18657274.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shin H, Iwasaki A (September 2013). "Tissue-resident memory T cells". Immunological Reviews. 255 (1): 165–81. doi:10.1111/imr.12087. PMC 3748618. PMID 23947354.
- ^ a b c https://medicalxpress.com/news/2017-03-highlights-achilles-heel-key-immune.html
- ^ Lee YJ, Jameson SC, Hogquist KA (February 2011). "Alternative memory in the CD8 T cell lineage". Trends in Immunology. 32 (2): 50–6. doi:10.1016/j.it.2010.12.004. PMC 3039080. PMID 21288770.
- ^ Marusina AI, Ono Y, Merleev AA, Shimoda M, Ogawa H, Wang EA, Kondo K, Olney L, Luxardi G, Miyamura Y, Yilma TD, Villalobos IB, Bergstrom JW, Kronenberg DG, Soulika AM, Adamopoulos IE, Maverakis E (February 2017). "+virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity" (PDF). Journal of Autoimmunity. 77: 76–88. doi:10.1016/j.jaut.2016.11.001. PMID 27894837.
- ^ White JT, Cross EW, Kedl RM (June 2017). "+T cells: where they come from and why we need them". Nature Reviews. Immunology. 17 (6): 391–400. doi:10.1038/nri.2017.34. PMC 5569888. PMID 28480897.
- ^ Hikono H, et al. (Jul 2007). "Activation phenotype, rather than central– or effector–memory phenotype, predicts the recall efficacy of memory CD8+ T cells". J Exp Med. 204 (7): 1625–1636. doi:10.1084/jem.20070322. PMC 2118640. PMID 17606632.
- ^ Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP (September 2011). "A human memory T cell subset with stem cell-like properties". Nature Medicine. 17 (10): 1290–7. doi:10.1038/nm.2446. PMC 3192229. PMID 21926977.
- ^ Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF (November 2005). "Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets". Journal of Immunology. 175 (9): 5895–903. doi:10.4049/jimmunol.175.9.5895. PMID 16237082.
- ^ a b Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R (March 2003). "Lineage relationship and protective immunity of memory CD8 T cell subsets". Nature Immunology. 4 (3): 225–34. doi:10.1038/ni889. PMID 12563257.
- ^ Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP (July 2005). "Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells". Proceedings of the National Academy of Sciences of the United States of America. 102 (27): 9571–6. doi:10.1073/pnas.0503726102. PMC 1172264. PMID 15980149.
- ^ Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG (December 2005). "Host-reactive CD8+ memory stem cells in graft-versus-host disease". Nature Medicine. 11 (12): 1299–305. doi:10.1038/nm1326. PMID 16288282.
- ^ a b Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP (July 2009). "Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells". Nature Medicine. 15 (7): 808–13. doi:10.1038/nm.1982. PMC 2707501. PMID 19525962.
- ^ Osborn JF, Mooster JL, Hobbs SJ, Munks MW, Barry C, Harty JT, Hill AB, Nolz JC (October 2017). "+T cells". Science Immunology. 2 (16): eaan6049. doi:10.1126/sciimmunol.aan6049. PMC 5786265. PMID 29030501.
Further reading
- Janeway, Charles (2005). Immunobiology: the immune system in health and disease. New York: Garland Science. ISBN 978-0-443-07310-6.
- Lichtman, Andrew H.; Abbas, Abul K. (2003). Cellular and molecular immunology. Philadelphia: Saunders. ISBN 0-7216-0008-5.
