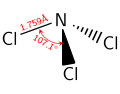Nitrogen trichloride
| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
Trichloramine
Agene Nitrogen(III) chloride Trichloroazane Trichlorine nitride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.029 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Cl3N | |||
| Molar mass | 120.36 g·mol−1 | ||
| Appearance | yellow oily liquid | ||
| Odor | chlorine-like | ||
| Density | 1.653 g/mL | ||
| Melting point | −40 °C (−40 °F; 233 K) | ||
| Boiling point | 71 °C (160 °F; 344 K) | ||
| immiscible slowly decomposes | |||
| Solubility | soluble in benzene, chloroform, CCl4, CS2, PCl3 | ||
| Structure | |||
| orthorhombic (below −40 °C) | |||
| trigonal pyramidal | |||
| 0.6 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
232 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| 93 °C (199 °F; 366 K) | |||
| Related compounds | |||
Other anions
|
Nitrogen trifluoride Nitrogen tribromide Nitrogen triiodide | ||
Other cations
|
Phosphorus trichloride Arsenic trichloride | ||
Related chloramines
|
Chloramine Dichloramine | ||
Related compounds
|
Nitrosyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine (for example, in swimming pools).
Preparation and structure
The compound is prepared by treatment of ammonium salts, such as ammonium nitrate with chlorine.
Intermediates in this conversion include chloramine and dichloramine, NH2Cl and NHCl2, respectively.
Like ammonia, NCl3 is a pyramidal molecule. The N-Cl distances are 1.76 Å, and the Cl-N-Cl angles are 107°.[1]
Nitrogen trichloride can form in small amounts when public water supplies are disinfected with monochloramine, and in swimming pools by disinfecting chlorine reacting with urea in urine from bathers. Nitrogen trichloride, trademarked as Agene, was used to artificially bleach and age flour, but was banned in 1949: In humans Agene was found to cause severe and widespread neurological disorders leading to its banning in 1947. Dogs that ate bread made from treated flour suffered epileptic-like fits; the toxic agent was methionine sulfoximine.[2]
Reactions
Despite the similarities of the Pauling electronegativities of nitrogen and chlorine, this molecule has moderate polarity with negative charges residing on nitrogen. So the nitrogen in NCl3 is often considered to have the +3 oxidation state and the chlorine atoms are considered to be in the -1 oxidation state.
It is hydrolyzed by hot water to release ammonia and hypochlorous acid.
- NCl3 + 3 H2O → NH3 + 3 HOCl
Safety
Nitrogen trichloride can irritate mucous membranes—it is a lachrymatory agent, but has never been used as such.[3][4] The pure substance (rarely encountered) is a dangerous explosive, being sensitive to light, heat, even moderate shock, and organic compounds. Pierre Louis Dulong first prepared it in 1812, and lost two fingers and an eye in two explosions. In 1813, an NCl3 explosion blinded Sir Humphry Davy temporarily, inducing him to hire Michael Faraday as a co-worker. They were both injured in another NCl3 explosion shortly thereafter.[5]
See also
References
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Shaw, CA; Bains, JS (1998). "Did consumption of flour bleached by the agene process contribute to the incidence of neurological disease?". Medical Hypotheses. 51 (6): 477–81. doi:10.1016/S0306-9877(98)90067-6. PMID 10052866.
- ^ White, G. C. (1999). The Handbook of Chlorination and Alternative Disinfectants (4th ed.). Wiley. p. 322. ISBN 978-0-471-29207-4.
- ^ "Health Hazard Evaluation Report: Investigation of Employee Symptoms at an Indoor Water Park" (pdf). NIOSH eNews. 6 (4). National Institute for Occupational Safety and Health. August 2008. HETA 2007-0163-3062.
- ^ Thomas, J.M. (1991). Michael Faraday and The Royal Institution: The Genius of Man and Place (PBK). CRC Press. p. 17. ISBN 978-0-7503-0145-9.
Further reading
- Jander, J. (1976). "Recent Chemistry and Structure Investigation of Nitrogen Triiodide, Tribromide, Trichloride, and Related Compounds". Advances in Inorganic Chemistry. 19: 1–63. doi:10.1016/S0065-2792(08)60070-9.
- Kovacic, P.; Lowery, M. K.; Field, K. W. (1970). "Chemistry of N-Bromamines and N-Chloramines". Chemical Reviews. 70 (6): 639–665. doi:10.1021/cr60268a002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hartl, H.; Schöner, J.; Jander, J.; Schulz, H. (1975). "Die Struktur des Festen Stickstofftrichlorids (−125 °C)". Zeitschrift für Anorganische und Allgemeine Chemie. 413 (1): 61–71. doi:10.1002/zaac.19754130108.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cazzoli, G.; Favero, P. G.; Dal Borgo, A. (1974). "Molecular Structure, Nuclear Quadrupole Coupling Constant and Dipole Moment of Nitrogen Trichloride from Microwave Spectroscopy". Journal of Molecular Spectroscopy. 50 (1–3): 82–89. doi:10.1016/0022-2852(74)90219-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bayersdorfer, L.; Engelhardt, U.; Fischer, J.; Höhne, K.; Jander, J. (1969). "Untersuchungen an Stickstoff–Chlor-Verbindungen. V. Infrarot- und RAMAN-Spektren von Stickstofftrichlorid". Zeitschrift für Anorganische und Allgemeine Chemie. 366 (3–4): 169–179. doi:10.1002/zaac.19693660308.
{{cite journal}}: CS1 maint: multiple names: authors list (link)