Octreotide scan
| Octreotide scan | |
|---|---|
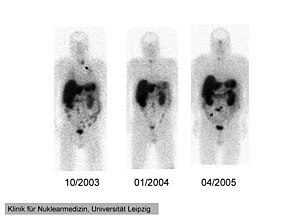 111In-pentetreotide scintigraphy of the 41-year-old man with ectopic Cushing' syndrome caused by a neuroendocrine carcinoma of the mesentery. Radiotracer accumulation in the left thyroid in 10/2003 (arrow). The mesenterial neuroendocrine tumor became clearly visible in 4/2005 (arrow). | |
| Synonyms | ocreoscan |
| ICD-9-CM | 92.18 |
| OPS-301 code | 3-70c |
An octreotide scan is a type of scintigraphy used to find carcinoid, pancreatic neuroendocrine tumors, and to localize sarcoidosis. It is also called somatostatin receptor scintigraphy (SRS). Octreotide, a drug similar to somatostatin, is radiolabeled with indium-111,[1] and is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin (i.e. gastrinoma, glucagonoma, etc). A gamma camera detects the radioactive octreotide, and makes pictures showing where the tumor cells are in the body.
Octreotide scanning is reported to have a sensitivity between 75% and 100% for detecting pancreatic neuroendocrine tumors.[2]
Indications
An octreotide scan may be used to locate suspected primary neuroendocrine tumours (NET) or for follow-up or staging after treatment.[3][4][5]
Procedure
The 111In-pentetreotide radiopharmaceutical is prepared from a kit in a radiopharmacy. Pentetreotide is a DTPA conjugate of octreotide.[3][6]
Approximately 200 megabecquerels of Indium-111 is injected intravenously. Imaging takes place 24 hours after injection, but may also be carried out at 4 and 48 hours.[4][7]
See also
References
- ^ medicinenet.com > Carcinoid Syndrome (cont.) By Dennis Lee and Jay Marks. Retrieved Mars 2011
- ^ Kwekkeboom, DJ; Krenning, EP (April 2002). "Somatostatin receptor imaging". Seminars in Nuclear Medicine. 32 (2): 84–91. doi:10.1053/snuc.2002.31022. PMID 11965603.
- ^ a b Kwekkeboom, Dik J.; Krenning, Eric P.; Scheidhauer, Klemens; Lewington, Val; Lebtahi, Rachida; Grossman, Ashley; Vitek, Pavel; Sundin, Anders; Plöckinger, Ursula (2009). "Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Somatostatin Receptor Imaging with 111In-Pentetreotide" (PDF). Neuroendocrinology. 90 (2): 184–189. doi:10.1159/000225946. PMID 19713709.
- ^ a b Bombardieri, Emilio; Ambrosini, Valentina; Aktolun, Cumali; Baum, Richard P.; Bishof-Delaloye, Angelica; Del Vecchio, Silvana; Maffioli, Lorenzo; Mortelmans, Luc; Oyen, Wim; Pepe, Giovanna; Chiti, Arturo (12 May 2010). "111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging" (PDF). European Journal of Nuclear Medicine and Molecular Imaging. 37 (7): 1441–1448. CiteSeerX 10.1.1.609.1835. doi:10.1007/s00259-010-1473-6. PMID 20461371.
- ^ Saha, Gopal B. (2010). Fundamentals of Nuclear Pharmacy. Springer. p. 145. ISBN 9781441958600.
- ^ "In 111 pentetreotide". NCI Drug Dictionary. National Cancer Institute. 2011-02-02. Retrieved 3 October 2017.
- ^ Balon, H. R.; Brown, T. L. Y.; Goldsmith, S. J.; Silberstein, E. B.; Krenning, E. P.; Lang, O.; Dillehay, G.; Tarrance, J.; Johnson, M.; Stabin, M. G. (8 November 2011). "The SNM Practice Guideline for Somatostatin Receptor Scintigraphy 2.0". Journal of Nuclear Medicine Technology. 39 (4): 317–324. doi:10.2967/jnmt.111.098277. PMID 22068564.
External links
- Octreotide scan entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
