Ovulation induction
| Ovulation induction | |
|---|---|
| Specialty | reproductive endocrinology and infertility, obstetrics |
| MeSH | D010062 |
Ovulation induction is the stimulation of ovulation by medication. It is usually used in the sense of stimulation of the development of ovarian follicles[1][2][3] to reverse anovulation or oligoovulation.
Scope
The term ovulation induction can potentially also be used for:
- Final maturation induction, in the sense of triggering oocyte release from relatively mature ovarian follicles during late follicular phase. In any case, ovarian stimulation (in the sense of stimulating the development of oocytes) is often used in conjunction with triggering oocyte release, such as for proper timing of artificial insemination.[4]
- Controlled ovarian hyperstimulation (stimulating the development of multiple follicles of the ovaries in one single cycle), has also appeared in the scope of ovulation induction.[4] Controlled ovarian hyperstimulation is generally part of in vitro fertilization, and the aim is generally to develop multiple follicles (optimally between 11 and 14 antral follicles measuring 2–8 mm in diameter),[5] followed by transvaginal oocyte retrieval, co-incubation, followed by embryo transfer of a maximum of two embryos at a time.[6]
- Also, where anovulation or oligovulation is secondary to another disease, the treatment for the underlying disease can be regarded as ovulation induction, by indirectly resulting in ovulation.
However, this article focuses on medical ovarian stimulation, during early to mid-follicular phase, without subsequent in vitro fertilization, with the aim of developing one or two ovulatory follicles (the maximum number before recommending sexual abstinence).[7]
Indications
Ovulation induction helps reversing anovulation or oligoovulation, that is, helping women who do not ovulate on their own regularly,[2] such as those with polycystic ovary syndrome (PCOS).[8]
Regimen alternatives
The main alternatives for ovulation induction medications are:
- Antiestrogen, causing an inhibition of the negative feedback of estrogen on the pituitary gland, resulting in an increase in secretion of follicle-stimulating hormone. Medications in use for this effect are mainly clomifene citrate and tamoxifen (both being selective estrogen-receptor modulators), as well as letrozole (an aromatase inhibitor.[citation needed]
- Follicle-stimulating hormone (FSH), directly stimulating the ovaries. In women with anovulation, it may be an alternative after 7 - 12 attempted cycles of antiestrogens (as evidenced by clomifene citrate), since the latter ones are less expensive and more easy to control.[9]
Antiestrogens

Clomifene citrate
Clomifene citrate (or clomid) is the medication which is most commonly used to treat anovulation. It is a selective estrogen-receptor modulator, affecting the hypothalamic–pituitary–gonadal axis to respond as if there was an estrogen deficit in the body, in effect increasing the production of follicle-stimulating hormone (FSH). It is relatively easy and convenient to use.[10] Clomifene appears to inhibit estrogen receptors in hypothalamus, thereby inhibiting negative feedback of estrogen on FSH production.[11] It may also result in direct stimulation of the hypothalamic-pituitary axis.[11] It also has an effect on cervical mucus quality and uterine mucosa, which might affect sperm penetration and survival, hence its early administration during the menstrual cycle. Clomifene citrate is a very efficient ovulation inductor, and has a success rate of 67%. Nevertheless, it only has a 37% success rate in inducing pregnancy. This difference may be due to the anti-estrogenic effect which clomifene citrate has on the endometrium, cervical mucus, uterine blood flow, as well as the resulting decrease in the motility of the fallopian tubes and the maturation of the oocytes.[12]
Letrozole
Letrozole has been used for ovarian stimulation by fertility doctors since 2001 because it has fewer side-effects than clomiphene and less chance of multiple gestation. A study of 150 babies following treatment with letrozole or letrozole and follicle-stimulating hormone presented at the American Society of Reproductive Medicine 2005 Conference found no difference in overall abnormalities but did find a significantly higher rate of locomotor and cardiac abnormalities among the group having taken letrozole compared to natural conception.[13] A larger, follow-up study with 911 babies compared those born following treatment with letrozole to those born following treatment with clomiphene.[14] That study also found no significant difference in the rate of overall abnormalities, but found that congenital cardiac anomalies was significantly higher in the clomiphene group compared to the letrozole group.
Tamoxifen
Tamoxifen affects estrogen receptors in a similar fashion as clomifene citrate. It is often used in the prevention and treatment of breast cancer. It can therefore also be used to treat patients that have a reaction to clomifene citrate.[15]
Follicle-stimulating hormone
Preparations of follicle-stimulating hormone (FSH) mainly include those derived from the urine of menopausal women, as well as recombinant preparations. The recombinant preparations are more pure and more easily administered, but they are more expensive. The urinary preparations are equally effective and less expensive, but are not as convenient to administer as they are available in vials versus injection pens.
GnRH pump
The gonadotropin-releasing hormone (GnRH) pump is used to release doses of GnRH in a pulsatile fashion. This hormone is synthesised by the hypothalamus and induces the secretion of FSH by the pituitary. GnRH must be delivered in a pulsatile fashion to imitate the random secretion of the hypothalamus in order to fool the pituitary into secreting LH and FSH. The GnRH pump is the size of a cigarette box and has a small catheter. Unlike other treatments, using the GnRH pump doesn’t usually lead to multiple pregnancies. Filicori from the University of Bologna suggests that this might be because gonadotrophins are absent when the treatment is initiated, and therefore the hormones released by the pituitary (LH and FSH) can still take part in the retro-control of gonadotrophin secretion, mimicking the natural cycle.[16] This treatment can also be used for underweight and/or anorexic patients;[17] it has also been used in certain cases of hyperprolactimenia.
National and regional usage
In the Nordic countries, letrozole is practically the standard initial regimen used for ovulation induction, since no formulation of clomifene is registered for use there.[18][19]
India banned the usage of letrozole in 2011, citing potential risks to infants.[20] In 2012, an Indian parliamentary committee said that the drug controller office colluded with letrozole's makers to approve the drug for infertility in India.[21]
Technique
Although there are many possible additional diagnostic and interventional techniques, protocols for ovulation induction generally consist of:
- Determining the first day of the last menstruation, which is termed day 1. In case of amenorrhea, a period can be induced by intake of an oral progestin for 10 days.
- Daily administration of the ovulation induction regimen, starting on day 3, 4, or 5,[22] and it is usually taken for 5 days.[9][23]
- Sexual intercourse or artificial insemination by the time of ovulation.
Ultrasonography
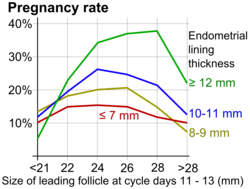
During ovulation induction, it is recommended to start at a low dose and monitor the ovarian response with transvaginal ultrasound, including discernment of the number of developing follicles. Initial exam is most commonly started 4–6 days after last pill. Serial transvaginal ultrasound can reveal the size and number of developing follicles. It can also provide presumptive evidence of ovulation such as sudden collapse of the preovulatory follicle, and an increase in fluid volume in the rectouterine pouch. After ovulation, it may reveal signs of luteinization such as loss of clearly defined follicular margins and appearance of internal echoes.
Supernumerary follicles
A cycle with supernumerary follicles is usually defined as one where there are more than two follicles >16 mm in diameter.[25] It is generally recommended to have such cycles cancelled because of the risk of multiple pregnancy (see also the "Risks and side effects" section below).[25][7] In cancelled cycles, the woman or couple should be warned of the risks in case of supernumerary follicles, and should avoid sexual intercourse or use contraception until the next menstruation.[25] Induction of final maturation (such as done with hCG) may need to be withheld because of increased risk of ovarian hyperstimulation syndrome(OHSS).[25] The starting dose of the inducing drug should be reduced in the next cycle.[25]
Alternatives to cancelling a cycle are mainly:
- Aspiration of supernumerary follicles until one or two remain.[25][26]
- Converting the protocol to IVF treatment with embryo transfer of up to two embryos only.[25]
- Selective fetal reduction. This alternative confers a high risk of complications.[25]
- Proceeding with any multiple pregnancy without fetal reduction, with the ensuing risk of complications. This alternative is not recommended.[25]
Lab tests
The following laboratory tests may be used to monitor induced cycles:[27]
- Serum estradiol levels, starting 4–6 days after last pill
- Post-coital test 1–3 days before ovulation to check whether there are at least 5 progressive sperm per HPF
- Adequacy of LH surge by urine LH surge tests 3 to 4 days after last clomifene pill
- Mid-luteal progesterone, with at least 10 ng/ml 7–9 days after ovulation being regarded as adequate.
Final maturation induction
Final maturation induction and release, such as by human chorionic gonadotropin (HCG or hCG) or recombinant luteinizing hormone (rLH), results in a predictable time of ovulation, with the interval from drug administration to ovulation depending on the type of drug. This avails for sexual intercourse or intrauterine insemination (IUI) to conviently be scheduled at ovulation, the most likely time to achieve pregnancy.[4]
As evidenced by clomifene-induced cycles, however, triggering oocyte release has been shown to decrease pregnancy chances compared to frequent monitoring with LH surge tests.[27] Therefore, in such cases, triggering oocyte release is best reserved for women who require IUI and in whom LH monitoring proves difficult or unreliable.[27] It may also be used when LH monitoring hasn't shown an LH surge by cycle day 18 (where cycle day 1 is the first day of the preseding menstruation) and there is an ovarian follicle of over 20 mm in size.[28]
Repeat cycles
Ovulation induction can be repeated every menstrual cycle. For clomifene, the dosage may be increased by 50-mg increments in subsequent cycles until ovulation is achieved.[27][29] However, at a dosage of 200 mg, further increments are unlikely to increase pregnancy chances.[27]
It is not recommended by the manufacturer of clomifene to use it for more than 6 consecutive cycles.[30][31] In women with anovulation, 7 - 12 attempted cycles of pituitary feedback regimens (as evidenced by clomifene citrate) are recommended before switching to gonadotrophins, since the latter ones are more expensive and less easy to control.[9]
It is no longer recommended to perform an ultrasound examination to exclude any significant residual ovarian enlargement before each new treatment cycle.[27]
Risks and side effects
Ultrasound and regular hormone checks mitigate risks throughout the process. However, there are still some risks with the procedure.
Ovarian hyperstimulation syndrome (OHSS) occurs in 5-10% of cases.[32] Symptoms depend on whether the case is mild, moderate, or severe, and can range from bloating and nausea, through to shortness of breathe, pleural effusion, and excessive weight gain (more than 2 pounds per day).
Multiple pregnancy
There is also the risk that more than one egg is produced, leading to twins or triplets. Women with polycystic ovary syndrome may be particularly at risk. Multiple pregnancy occurs in approximately 15-20% of cases following cycles induced with gonadotrophins such as hMG and FSH induced ovulations.[25] The risks associated with multiple pregnancy are much higher than singleton pregnancy; incidences of perinatal death are seven times higher in triplet births and five times higher in twin births than the risks associated with a singleton pregnancy.[33][34] It is therefore important to adapt the treatment to each individual patient.[35] If more than one or two ovulatory follicles are detected on ultrasonography, sexual abstinence is recommended.[7]
Alternatives
- In vitro fertilization, including controlled ovarian hyperstimulation.
- In vitro maturation is letting ovarian follicles mature in vitro, and this technique can potentially be an alternative both to anovulation reversal and oocyte release triggering. Rather, oocytes can mature outside the body, such as prior to IVF. Hence, no (or at least a lower dose of) gonadotropins have to be injected in the body.[36] However, there still isn't enough evidence to prove the effectiveness and security of the technique.[36]
References
- ^ Ovulation Problems and Infertility: Treatment of ovulation problems with Clomid and other fertility drugs. Advanced Fertility Center of Chicago. Gurnee & Crystal Lake, Illinois. Retrieved on Mars 7, 2010
- ^ a b Flinders reproductive medicine > Ovulation Induction Archived 2009-10-03 at the Wayback Machine Retrieved on Mars 7, 2010
- ^ fertilityLifeLines > Ovulation Induction Archived 2013-03-10 at the Wayback Machine Retrieved on Mars 7, 2010
- ^ a b c IVF.com > Ovulation Induction Archived 2012-02-26 at the Wayback Machine Retrieved on Mars 7, 2010
- ^ Antral Follicle Counts, Resting Follicles, Ovarian Volume and Ovarian Reserve. Testing of egg supply and predicting response to ovarian stimulation drugs Advanced Fertility Center of Chicago. Retrieved on October 2, 2009
- ^ Fertility: assessment and treatment for people with fertility problems. NICE clinical guideline CG156 - Issued: February 2013
- ^ a b c "Ovulation Induction". Manchester University. Retrieved 2019-04-04.
- ^ Ovulation Problems and Infertility: Treatment of ovulation problems with Clomid and other fertility drugs Advanced Fertility Center of Chicago. Gurnee & Crystal Lake, Illinois
- ^ a b c Weiss, N. S.; Braam, S.; Konig, T. E.; Hendriks, M. L.; Hamilton, C. J.; Smeenk, J. M. J.; Koks, C. A. M.; Kaaijk, E. M.; Hompes, P. G. A.; Lambalk, C. B.; van der Veen, F.; Mol, B. W. J.; van Wely, M. (2014). "How long should we continue clomiphene citrate in anovulatory women?". Human Reproduction. 29 (11): 2482–2486. doi:10.1093/humrep/deu215. ISSN 0268-1161. PMID 25164024.
- ^ Lord JM, Flight IH, Norman RJ (October 2003). "Metformin in polycystic ovary syndrome: systematic review and meta-analysis". BMJ. 327 (7421): 951–0. doi:10.1136/bmj.327.7421.951. PMC 259161. PMID 14576245.
- ^ a b DrugBank > Clomifene. Updated on April 19, 2011
- ^ Kousta E, White DM, Franks S (1997). "Modern use of clomiphene citrate in induction of ovulation". Hum. Reprod. Update. 3 (4): 359–365. doi:10.1093/humupd/3.4.359. PMID 9459281.
- ^ Biljan MM, Hemmings R, Brassard N (2005). "The Outcome of 150 Babies Following the Treatment With Letrozole or Letrozole and Gonadotropins". Fertility and Sterility. 84: S95. doi:10.1016/j.fertnstert.2005.07.230.
- ^ Tulandi T, Martin J, Al-Fadhli R, et al. (June 2006). "Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate". Fertility and Sterility. 85 (6): 1761–5. doi:10.1016/j.fertnstert.2006.03.014. PMID 16650422.
- ^ Boostanfar R, Jain JK, Mishell DR, Paulson RJ (May 2001). "A prospective randomized trial comparing clomiphene citrate with tamoxifen citrate for ovulation induction". Fertil. Steril. 75 (5): 1024–1026. doi:10.1016/S0015-0282(01)01749-6. PMID 11334921.
- ^ Filicori M, Flamigni C, Dellai P, et al. (October 1994). "Treatment of anovulation with pulsatile gonadotropin-releasing hormone: prognostic factors and clinical results in 600 cycles". J. Clin. Endocrinol. Metab. 79 (4): 1215–1220. doi:10.1210/jc.79.4.1215. PMID 7962297.
- ^ Braat DD, Schoemaker R, Schoemaker J (February 1991). "Life table analysis of fecundity in intravenously gonadotropin-releasing hormone-treated patients with normogonadotropic and hypogonadotropic amenorrhea". Fertil. Steril. 55 (2): 266–71. doi:10.1016/S0015-0282(16)54113-2. PMID 1991525.
- ^ "Pergotime avregistreres 31.12.2016". Statens legemiddelverk. 2016-09-09.
- ^ "Pergotime". FASS.
- ^ Sinha, Kounteya (18 October 2011). "Finally, expert panel bans fertility drug Letrozole". The Times of India. Retrieved 14 November 2011.
- ^ "House panel to govt: Punish those guilty of approving Letrozole". The Times of India. 10 April 2007. Retrieved 9 May 2012.
- ^ Emre Seli, Aydin Arici. "Patient education: Ovulation induction with clomiphene (Beyond the Basics)". UpToDate. Topic last updated: Aug 01, 2017
- ^ Robert F Casper. "Ovulation induction with letrozole". UpToDate. Topic last updated: Sep 17, 2018.
- ^ Palatnik, Anna; Strawn, Estil; Szabo, Aniko; Robb, Paul (2012). "What is the optimal follicular size before triggering ovulation in intrauterine insemination cycles with clomiphene citrate or letrozole? An analysis of 988 cycles". Fertility and Sterility. 97 (5): 1089–1094.e3. doi:10.1016/j.fertnstert.2012.02.018. ISSN 0015-0282. PMID 22459633.
- ^ a b c d e f g h i j Hong Kong College of Obstetricians and Gynaecologists > Guidelines for use of gonadotrophins- revised Archived September 9, 2012, at the Wayback Machine. Number 1. April 2003.
- ^ Albano, C.; Platteau, P.; Nogueira, D.; Cortvrindt, R.; Smitz, J.; Devroey, P. (2001). "Avoidance of multiple pregnancies after ovulation induction by supernumerary preovulatory follicular reduction". Fertility and Sterility. 76 (4): 820–822. doi:10.1016/S0015-0282(01)02379-2. PMID 11591420.
- ^ a b c d e f Practice Committee of the American Society for Reproductive Medicine (August 2013). "Use of clomiphene citrate in infertile women: a committee opinion". Fertil. Steril. 100 (2): 341–8. doi:10.1016/j.fertnstert.2013.05.033. PMID 23809505.
- ^ Clomiphene Citrate, Clomid Archived 2014-05-10 at archive.today. By Robert B. McWilliams. The Center for Reproduction and Women's Health Care, Houston, Texas. Retrieved May 2014
- ^ Medications for Inducing Ovulation from American Society for Reproductive Medicine. Revised 2012.
- ^ "Clomiphene citrate tablets label" (PDF). Revised October 2012: FDA. Archived (PDF) from the original on September 27, 2016. Retrieved September 11, 2016.
{{cite web}}: CS1 maint: location (link) - ^ Trabert, B.; Lamb, E. J.; Scoccia, B.; Moghissi, K. S.; Westhoff, C. L.; Niwa, S.; Brinton, L. A. (2013). "Ovulation-inducing drugs and ovarian cancer risk: Results from an extended follow-up of a large US infertility cohort". Fertility and Sterility. 100 (6): 1660–6. doi:10.1016/j.fertnstert.2013.08.008. PMC 3873340. PMID 24011610.
- ^ Ovulation Induction Risks and Overview
- ^ Bergh T, Ericson A, Hillensjö T, Nygren KG, Wennerholm UB (November 1999). "Deliveries and children born after in-vitro fertilisation in Sweden 1982-95: a retrospective cohort study". Lancet. 354 (9190): 1579–1585. doi:10.1016/S0140-6736(99)04345-7. PMID 10560671. S2CID 11057942.
- ^ Fisk NM, Trew G (November 1999). "Two's company, three's a crowd for embryo transfer". Lancet. 354 (9190): 1572–1573. doi:10.1016/S0140-6736(99)00290-1. PMID 10560665. S2CID 37575727.
- ^ Eshre Capri Workshop Group (2003). "Mono-ovulatory cycles: a key goal in profertility programmes". Hum. Reprod. Update. 9 (3): 263–274. doi:10.1093/humupd/dmg020. PMID 12859047.
- ^ a b "Vejledning om kunstig befrugtning 2006 (Danish)" (PDF). Archived from the original (PDF) on 2012-03-09. Retrieved 2011-09-25.
