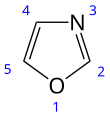
Oxazole
Appearance
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-oxazole
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.474 | ||
| EC Number |
| ||
| MeSH | D010080 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H3NO | |||
| Molar mass | 69.06 g/mol | ||
| Density | 1.050 g/cm3 | ||
| Boiling point | 69 to 70 °C (156 to 158 °F; 342 to 343 K) | ||
| Acidity (pKa) | 0.8 (of conjugate acid) [1] | ||
| Supplementary data page | |||
| Oxazole (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon.[2] Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Preparation
Classical oxazole synthetic methods in organic chemistry are
- the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones
- the Fischer oxazole synthesis from cyanohydrins and aldehydes
- the Bredereck reaction with α-haloketones and formamide
- the Van Leusen reaction with aldehydes and TosMIC
Other methods are reported in literature.
- Oxazolines can also be obtained from cycloisomerization of certain propargyl amides. In one study[3] oxazoles were prepared via a one-pot synthesis consisting of the condensation of propargyl amine and benzoyl chloride to the amide, followed by a Sonogashira coupling of the terminal alkyne end with another equivalent of benzoylchloride, and concluding with p-toluenesulfonic acid catalyzed cycloisomerization:
- In one reported oxazole synthesis the reactants are a nitro-substituted benzoyl chloride and an isonitrile:[4][5]
Biosynthesis
In biomolecules, oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides:

Where X = H, CH
3 for serine and threonine respectively, B = base.
(1) Enzymatic cyclization. (2) Elimination. (3) [O] = enzymatic oxidation.
Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom.
Reactions
- Deprotonation of oxazoles at C2 is often accompanied by ring-opening to the isonitrile.
- Electrophilic aromatic substitution takes place at C5 requiring activating groups.
- Nucleophilic aromatic substitution takes place with leaving groups at C2.
- Diels–Alder reactions with oxazole dienes can be followed by loss of oxygen to form pyridines.
- The Cornforth rearrangement of 4-acyloxazoles is a thermal rearrangement reaction with the organic acyl residue and the C5 substituent changing positions.
- Various oxidation reactions. One study[6] reports on the oxidation of 4,5-diphenyloxazole with 3 equivalents of CAN to the corresponding imide and benzoic acid:
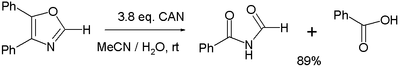
- In the balanced half-reaction three equivalents of water are consumed for each equivalent of oxazoline, generating 4 protons and 4 electrons (the latter derived from CeIV).
See also
- Isoxazole, an analog with the nitrogen atom in position 2.
- Imidazole, an analog with the oxygen replaced by a nitrogen.
- Thiazole, an analog with the oxygen replaced by a sulfur.
- Benzoxazole, where the oxazole is fused to another aromatic ring.
- Pyrrole, an analog without the oxygen atom.
- Furan, an analog without the nitrogen atom.
- Oxazoline, which has one double bond reduced.
- Oxazolidine, which has both double bonds reduced.
- Oxadiazoles with two nitrogens instead of one (e.g. furazan).
- Oxazolone, an analog with a carbonyl group
References
- ^ Zoltewicz, J. A. & Deady, L. W. Quaternization of heteroaromatic compounds. Quantitative aspects. Adv. Heterocycl. Chem. 22, 71-121 (1978).
- ^ Heterocyclic Chemistry TL Gilchrist, The Bath press 1985 ISBN 0-582-01421-2
- ^ A new consecutive three-component oxazole synthesis by an amidation–coupling–cycloisomerization (ACCI) sequence Eugen Merkul and Thomas J. J. Müller Chem. Commun., 2006, 4817 - 4819, doi:10.1039/b610839c
- ^ Fully Automated Continuous Flow Synthesis of 4,5-Disubstituted Oxazoles Marcus Baumann, Ian R. Baxendale, Steven V. Ley, Christoper D. Smith, and Geoffrey K. Tranmer Org. Lett.; 2006; 8(23) pp 5231 - 5234; (Letter) doi:10.1021/ol061975c
- ^ They react together in the first phase in a continuous flow reactor to the intermediate enol and then in the second phase in a phosphazene base (PS-BEMP) induced cyclization by solid-phase synthesis.
- ^ Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles David A. Evans, Pavel Nagorny, and Risheng Xu Org. Lett.; 2006; 8(24) pp 5669 - 5671; (Letter) doi:10.1021/ol0624530